- Desmethylprodine
-
"MPPP" redirects here. For other uses, see MPPP (disambiguation).
Desmethylprodine 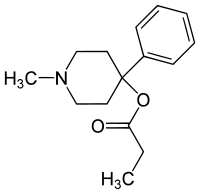
Systematic (IUPAC) name (1-methyl-4-phenylpiperidin-4-yl) propanoate Clinical data Pregnancy cat. ? Legal status Schedule I (US) Identifiers CAS number 13147-09-6 ATC code ? PubChem CID 61583 DrugBank DB01478 ChemSpider 55493 
ChEMBL CHEMBL279865 
Synonyms 4-propionyloxy-4-phenyl-N-methylpiperidine, MPPP, 3-desmethylprodine Chemical data Formula C15H21NO2 Mol. mass 247.33 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)1-Methyl-4-phenyl-4-propionoxypiperidine (MPPP) or Desmethylprodine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche[1]. It is not used in clinical practice, but has been illegally manufactured for recreational drug use. It is an analog of meperidine (Demerol), but, since it is not used in medicine, the DEA has labeled it a Schedule I drug in the United States. In fact, it is the reversed ester of pethidine and is listed as having 70% of the potency of morphine.
The drug was first synthesised in 1977 for recreational purposes by a 23-year old graduate student named Barry Kidston. Kidston had studied a 1947 paper by Albert Ziering.[2] By reversing the ester of the meperidine skeleton, a then-legally uncontrolled drug approaching the potency of morphine was produced.
However, the intermediate tertiary alcohol is liable to dehydration in acidic conditions if the reaction temperature rises above -30°C, and, since Kidston did not realize this and esterified the intermediate with propionic anhydride at room temperature, MPTP was formed as a major impurity.[3] Several days after trying this new batch of his homemade drug, Kidston developed serious Parkinson's Disease symptoms, as did several friends he had shared the drug with.[4]
1-Methyl-4-phenylpyridinium (MPP+), a metabolite of MPTP, causes rapid onset of irreversible symptoms similar to Parkinson's Disease.[5][6] MPTP is metabolized to the neurotoxin MPP+ by the enzyme MAO-B, which is expressed in glial cells. This selectively kills brain tissue in the area of the brain called the substantia nigra and causes Parkinsonian symptoms.[7]
References
- ^ US Patent 2498432 - I. Branched Lower Alkyl-4-Phenyl-4-Aceloxy Piperidines
- ^ Ziering, A.; Lee, J. (1947). "Piperidine derivatives; 1,3-dialkyl-4-aryl-4-acyloxypiperidines". The Journal of organic chemistry 12 (6): 911–914. doi:10.1021/jo01170a024. PMID 18919744.
- ^ Johannessen, J. N.; Markey, S. P. (1984). "Assessment of the opiate properties of two constituents of a toxic illicit drug mixture". Drug and alcohol dependence 13 (4): 367–374. doi:10.1016/0376-8716(84)90004-8. PMID 6148225.
- ^ Gibb, Barry J. (2007). The Rough Guide to the Brain, Rough Guides Ltd., London, pg.166
- ^ Davis, G. C.; Williams, A. C.; Markey, S. P.; Ebert, M. H.; Caine, E. D.; Reichert, C. M.; Kopin, I. J. (1979). "Chronic Parkinsonism secondary to intravenous injection of meperidine analogues". Psychiatry research 1 (3): 249–254. doi:10.1016/0165-1781(79)90006-4. PMID 298352.
- ^ Wallis, Claudia (2001-06-24). "Surprising Clue to Parkinson's - TIME". Time. http://www.time.com/time/magazine/article/0,9171,1101850408-141542,00.html. Retrieved 2010-05-13.
- ^ Schmidt, N.; Ferger, B. (2001). "Neurochemical findings in the MPTP model of Parkinson's disease". Journal of neural transmission (Vienna, Austria : 1996) 108 (11): 1263–1282. doi:10.1007/s007020100004. PMID 11768626.

This analgesic-related article is a stub. You can help Wikipedia by expanding it.
