- Pseudomorphine
-
"Dehydromorphine" redirects here. It is not to be confused with dihydromorphine.
Pseudomorphine 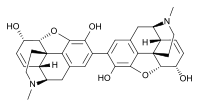 (5α,6α)-2-[(5α,6α)-3,6-dihydroxy-17-methyl-7,8-didehydro-4,5-epoxymorphinan-2-yl]-17-methyl-7,8-didehydro-4,5-epoxymorphinan-3,6-diol
(5α,6α)-2-[(5α,6α)-3,6-dihydroxy-17-methyl-7,8-didehydro-4,5-epoxymorphinan-2-yl]-17-methyl-7,8-didehydro-4,5-epoxymorphinan-3,6-diolIdentifiers Abbreviations 2,2'-bimorphine[1] PubChem 234570 ChemSpider 4590027 
UNII AEZ78QX2G7 Jmol-3D images Image 1 - CN1CCC23C4C1CC5=C2C(=C(C(=C5)C6=CC7=C8C(=C6O)OC9C81CCN(C(C7)C1C=CC9O)C)O)OC3C(C=C4)O
- InChI=1S/C34H36N2O6/c1-35-9-7-33-19-3-5-23(37)31(33)41-29-25(33)15(13-21(19)35)11-17(27(29)39)18-12-16-14-22-20-4-6-24(38)32-34(20,8-10-36(22)2)26(16)30(42-32)28(18)40/h3-6,11-12,19-24,31-32,37-40H,7-10,13-14H2,1-2H3
Key: FOJYFDFNGPRXDR-UHFFFAOYSA-N
Properties Molecular formula C34H36N2O6 Molar mass 568.66 g mol−1 Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox references Pseudomorphine (also known as oxydimorphine or dehydromorphine) is a natural dimerisation product of the morphine molecule in tandem and thus a common impurity in morphine concentrations. It was first described by Pelletier in 1835.[2]
This compound may be synthesized by the oxidative coupling of morphine by potassium ferricyanide.[1]
See also
References
- ^ a b Bentley, K. W.; Dyke, S. F. (1959). "512. The structure of pseudomorphine". J. Chem. Soc.: 2574. doi:10.1039/JR9590002574.
- ^ A. K. Balls (1927). "Concerning Pseudomorphine". J. Biol. Chem 71: 537-542. http://www.jbc.org/content/71/2/537.

This analgesic-related article is a stub. You can help Wikipedia by expanding it.
