- Dermorphin
-
Dermorphin 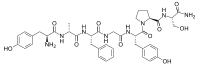 Other namesTyrosyl-alanyl-phenylalanyl-glycyl-tyrosyl-prolyl-serinamide
Other namesTyrosyl-alanyl-phenylalanyl-glycyl-tyrosyl-prolyl-serinamideIdentifiers CAS number 77614-16-5 
PubChem 5485199 ChEMBL CHEMBL278789 
Jmol-3D images Image 1 - C[C@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N3CCC[C@H]3C(=O)N[C@@H](CO)C(=O)N)NC(=O)[C@H](CC4=CC=C(C=C4)O)N
Properties Molecular formula C40H50N8O10 Molar mass 802.87 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dermorphin is a hepta-peptide first isolated from the skin of South American frogs belonging to the genus Phyllomedusa.[1] The peptide is a natural opioid that binds as an agonist with high potency and selectivity to mu Opioid receptors.[2][3] Dermorphin is about 30-40 times more potent than morphine but less likely to produce drug tolerance and addiction.[4] The amino acid sequence of demorphin is H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2
Dermorphin is not found in humans or other mammals and similar D-amino acid peptides have only been found in bacteria, amphibians and molluscs.[5] Dermorphin appears to be made in these through an unusual posttranslational modification carried out by an amino acid isomerase.[6] The reason why such an unusual process is needed is because the D-alanine in this peptide is not among the 20 in the genetic code and cannot be encoded in the genes by higher organisms.
References
- ^ Melchiorri P, Negri L (1996). "The dermorphin peptide family". Gen. Pharmacol. 27 (7): 1099–107. doi:10.1016/0306-3623(95)02149-3. PMID 8981054.
- ^ Amiche M, Delfour A, Nicolas P (1998). "Opioid peptides from frog skin". EXS 85: 57–71. PMID 9949868.
- ^ Erspamer V, Melchiorri P, Falconieri-Erspamer G, et al. (1989). "Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites". Proc. Natl. Acad. Sci. U.S.A. 86 (13): 5188–92. doi:10.1073/pnas.86.13.5188. PMC 297583. PMID 2544892. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=297583.
- ^ Broccardo M, Erspamer V, Falconieri Erspamer G, et al. (1981). "Pharmacological data on dermorphins, a new class of potent opioid peptides from amphibian skin". Br. J. Pharmacol. 73 (3): 625–31. PMC 2071698. PMID 7195758. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2071698.
- ^ Kreil G (15 April 1994). "Peptides containing a D-amino acid from frogs and molluscs". J. Biol. Chem. 269 (15): 10967–70. PMID 8157620. http://www.jbc.org/cgi/reprint/269/15/10967.
- ^ Heck SD, Faraci WS, Kelbaugh PR, Saccomano NA, Thadeio PF, Volkmann RA (1996). "Posttranslational amino acid epimerization: enzyme-catalyzed isomerization of amino acid residues in peptide chains". Proc. Natl. Acad. Sci. U.S.A. 93 (9): 4036–9. doi:10.1073/pnas.93.9.4036. PMC 39482. PMID 8633012. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=39482.
Categories:- Opioids
- Mu-opioid agonists
- Peptides
Wikimedia Foundation. 2010.
