- PEPAP
-
PEPAP 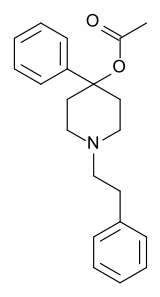
Systematic (IUPAC) name 4-phenyl-1-(2-phenylethyl)piperidin-4-yl acetate Clinical data Pregnancy cat. ? Legal status ? (UK) Schedule I (US) Identifiers CAS number 64-52-8 ATC code ? PubChem CID 60977 DrugBank DB01562 ChemSpider 54939 
Synonyms PEPAP Chemical data Formula C21H25NO2 Mol. mass 323.43 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)PEPAP is an opioid analgesic that is an analog of pethidine (meperidine).
It is related to the drug MPPP, with an N-phenethyl group in place of the N-methyl substitution and an acetate ester rather than propionate. PEPAP is approximately 6-7 times more potent than morphine in laboratory rats.[1] PEPAP presumably has similar effects to other opioids, producing analgesia, sedation and euphoria. Side effects can include itching, nausea and potentially serious respiratory depression which can be life-threatening.
PEPAP has been found to be a potent CYP2D6 inhibitor, which makes it likely to cause adverse interactions with some other drugs, although the inhibitory potency of PEPAP is less than that of MPPP.[2] (Both cocaine and methadone are also CYP2D6 inhibitors and could, in theory, potentiate the effect.)
It is unlikely that the tetrahydropyridine byproducts that may be formed during the synthesis of PEPAP are neurotoxic in the same way as the MPPP byproduct MPTP. It appears that the N-methyl group of MPTP is required for neurotoxic activity. In animal experiments, only MPTP analogues that preserved the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine structure were active as dopaminergic neurotoxins. Most structural changes, including replacing the N-methyl group with other substituents, abolished neurotoxicity.[3]
There is evidence that the clandestine manufacturers who produced MPPP in the 1970s (included the tainted batch) went on to produce PEPAP[4] in an attempt to avoid using watched precursors or drug intermediates that were illegal.
References
- ^ Janssen, PA; Eddy, NB (1960). "Compounds related to pethidine-IV. New general chemical methods of increasing the analgesic activity of pethidine". Journal of medicinal and pharmaceutical chemistry 2: 31–45. doi:10.1021/jm50008a003. PMID 14406754.
- ^ Pritzker, D; Kanungo, A; Kilicarslan, T; Tyndale, RF; Sellers, EM (2002). "Designer drugs that are potent inhibitors of CYP2D6". Journal of clinical psychopharmacology 22 (3): 330–2. PMID 12006905.
- ^ Youngster, SK; Sonsalla, PK; Sieber, BA; Heikkila, RE (1989). "Structure-activity study of the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity. I. Evaluation of the biological activity of MPTP analogs". The Journal of pharmacology and experimental therapeutics 249 (3): 820–8. PMID 2786564.
- ^ The Case of the Frozen Addicts (ISBN 0-679-42465-2)
Categories:- Piperidines
- Acetate esters
- Mu-opioid agonists
- Synthetic opioids
Wikimedia Foundation. 2010.
