- Difenoxin
-
Further information: Diphenoxylate
Difenoxin 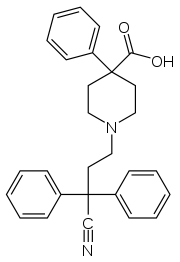
Systematic (IUPAC) name 1-(3-cyano-3,3-diphenylpropyl)-4-phenylpiperidine-4-carboxylic acid Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status Schedule IV (US) Identifiers CAS number 35607-36-4 
ATC code A07DA04 PubChem CID 34328 DrugBank DB01501 ChemSpider 31620 
UNII 3ZZ5BJ9F2Q 
KEGG D03809 
ChEBI CHEBI:4534 
ChEMBL CHEMBL1201321 
Synonyms Difenoxin, Motofen Chemical data Formula C28H28N2O2 Mol. mass 424.53 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Difenoxin (Motofen, R-15403) is a 4-phenylpiperidine derivative that is related to the opioid analgesic drug pethidine (meperidine) and more distantly related to alphaprodine and piritramide, and it is an active metabolite of the anti-diarrhoea drug diphenoxylate. Difenoxin et al. have a high peripheral/central actions ratio, working primarily on various opioid receptors in the intestines.[1] Difenoxin was developed in 1970 in Belgium at Janssen Pharmaceutica.
Contents
Related compounds
Loperamide (Imodium) is also closely related to difenoxin in chemical structure but does not cross the blood-brain barrier as does difenoxin, and the combination drug diphenoxylate hydrochloride with atropine sulphate (Lomotil) is the prototype of this four-member subfamily of the synthetic opioid receptor agonists and sub-category of opioid anti-diarrhoeals. Pethidine was serendipitiously discovered in research during the 1930s on gastrointestinal drugs to serve as alternatives to belladonna and opium derivatives, but the anti-diarrhoeal effects of pethidine are less than those of this subclass and equivalent contstipating doses of pethidine have prominent central actions. Therefore, with difenoxin and related drugs, the ratio of GI effects to central narcotic effects is particularly high, which makes it an attractive alternative in the minds of many to the other opioids used for diarrhoea, viz. codeine, morphine, dihydrocodeine, paregoric, laudanum and opium . The parent of the three above-mentioned pethidine-related anti-diarrhoeals is diphenoxylic acid, which can also be manufactured and used pharmaceutically.
Legal status
Difenoxin is a Schedule I drug by itself (Lyspafen) in the USA.[2] It has been approved for use in the late 1990s in the form of Motofen (difenoxin HCl and atropine tablets and elixir) which like Lomotil is a less-restrictive category Schedule IV on account of the adulterant; one difference is that the diphenoxylate and atropine formulation is Schedule V. Atropine is present in each doseage unit in the amount of 25 µg, or 1/40 of the therapeutic dose. Many other countries have been using this combination product for many years as a second-line centrally-acting and/or opioid-agonist anti-diarrhoeal, betwixt loperamide and paregoric.[3]
Side effects
Diarrhoea resulting from cyclic or diarrhoea-predominant IBS may not be optimally treated with diphenoxylate or difenoxin, and may not respond to a meaningful degree to loperamide; thus, diarrhoea and cramping which does not respond to belladonna derivatives and non-centrally-acting soothing and/or stool-desiccating agents are often treated with conservative doses of codeine, especially where paregoric and/or laudanum are not currently in general use.
Difenoxin also has some sedative and analgesic effects as with other opioids, but diphenoxylate itself is a relatively weak analgesic, and difenoxin has similarly limited analgesic effects, although it is a potent anti-diarrheal drug. Research suggests that additional non-opioid mechanisms may also be involved in the action of difenoxin, explaining its strong anti-diarrheal effects despite only limited opioid action.[4]
References
- ^ Jackson LS, Stafford JE. The evaluation and application of a radioimmunoassay for the measurement of diphenoxylic acid, the major metabolite of diphenoxylate hydrochloride (Lomotil), in human plasma. Journal of Pharmacological Methods. 1987 Nov;18(3):189-97.
- ^ http://www.deadiversion.usdoj.gov/schedules/orangebook/orangebook.pdf
- ^ Innocenti P, Rossi L, Bombardieri G. Clinical effectiveness of difenoxine in patients with acute and chronic diarrhea. (Italian). Bollettino Chimico Farmaceutico. 1983 Dec;122(12):64S-68S.
- ^ De Luca A, Coupar IM. Difenoxin and loperamide: studies on possible mechanisms of intestinal antisecretory action. Naunyn Schmiedebergs Archives of Pharmacology. 1993 Feb;347(2):231-7.
Categories:- Synthetic opioids
- Nitriles
- Piperidines
- Antidiarrhoeals
- Mu-opioid agonists
Wikimedia Foundation. 2010.
