- 6-Monoacetylcodeine
-
6-Monoacetylcodeine 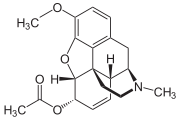
Systematic (IUPAC) name 3-methoxy-6-acetyl-(5α,6α)-7,8-didehydro-4,5-epoxy- Clinical data Pregnancy cat. ? Legal status ? Routes Intravenous (if present in heroin) Identifiers CAS number 6703-27-1 
ATC code None PubChem CID 5745725 ChemSpider 4588962 
Synonyms 6-acetylcodeine Chemical data Formula C20H23NO4 Mol. mass 341.4 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)6-Monoacetylcodeine (6-MAC) is a toxic acetate ester of codeine in which the hydroxyl group on the 6 position has been acetylated. It is occasionally present as an impurity in street heroin and is typically created when attempting to create heroin from a solution of morphine in which some of the codeine from the original opium solution still remains. It is formed either through the addition of acetic anhydride, which will only acetylate the 6 position on the codeine or as a result of the addition of acetic acid with a catalyst in an attempt to create 6-monoacetylmorphine, the equivalent ester of morphine which is slightly more potent than heroin itself. Administration of 6-MAC through any route results in the rapid release of histamine into the blood stream which can lead to potentially fatal anaphylactic shock. 6-monoacetylcodeine is eventually metabolised into codeine and then into morphine. Since only illegally produced heroin is likely to contain 6-MAC, testing for the presence of it in the urine can be used as a fairly reliable method of detecting the use of illicit heroin, as opposed to prescription painkillers. [1]
See also
References
External links

This analgesic-related article is a stub. You can help Wikipedia by expanding it.
