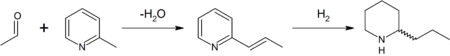- Coniine
-
Coniine 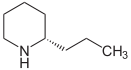
 (2S)-2-propylpiperidine
(2S)-2-propylpiperidineIdentifiers CAS number 458-88-8 
PubChem 441072 ChemSpider 389878 
UNII C479P32L2D 
KEGG C06523 
ChEBI CHEBI:28322 
Jmol-3D images Image 1 - N1[C@@H](CCC)CCCC1
Properties Molecular formula C8H17N Molar mass 127.23 g mol−1 Melting point -2 °C, 271 K, 28 °F
Boiling point 166-167 °C, 439-440 K, 331-333 °F
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Coniine is a poisonous alkaloid found in poison hemlock and the yellow pitcher plant, and contributes to hemlock's fetid smell. It is a neurotoxin which disrupts the peripheral nervous system. It is toxic to humans and all classes of livestock; less than 0.2g is fatal to humans, with death caused by respiratory paralysis. Socrates was put to death by way of this poison in 399 BC. Coniine has two stereoisomers: (S)-(+)-coniine (CAS 458-88-8), which is the natural isomer present in hemlock and (R)-(-)-coniine (CAS 5985-99-9). Coniine was first synthesized by Albert Ladenburg in 1886; it was the first of the alkaloids to be synthesized.
Contents
Isolation and properties
This alkaloid was first isolated by Giesecke,[1] but the formula was suggested by Blyth[2] and definitely established by Hoffmann.[3] D-(S)-Coniine is a colourless alkaline liquid, with a penetrating odour and a burning taste; has D0° 0.8626 and D19° 0.8438, refractive index n23°D 1.4505, and is dextrorotatory, [α]19°D +15.7°. It solidifies into a soft crystalline mass at -2 °C. Coniine is slightly soluble (1 in 90) in cold water, less so in hot water, so that a clear cold solution becomes turbid when warmed. On the other hand, the base dissolves about 25% of water at room temperature. It mixes with alcohol in all proportions, is readily soluble in ether and most organic solvents. Coniine slowly oxidises in the air. The salts crystallise well and are soluble in water or alcohol. The hydrochloride, B•HCl, crystallises from water in rhombs, mp. 220 °C, [α]20°D +10.1°; the hydrobromide, in needles, mp. 211 °C, and the D-acid tartrate, B•C4H6O6•2 H2O, in rhombic crystals, mp. 54 °C. The platinichloride, (B•HCl)2•PtCl4•H2O, separates from concentrated solution as an oil, which solidifies to a mass of orange-yellow crystals, mp. 175 °C (dry). The aurichloride, B•HAuCl4, crystallises on standing, mp. 77 °C. The picrate forms small yellow needles, mp. 75 °C, from hot water. The 2,4-dinitrobenzoyl- and 3,5-dinitrobenzoyl-derivates have mps. 139.0-139.5 °C and 108-9 °C respectively.[4] Coniine dissolves in carbon disulfide, forming a complex thiocarbamate.[5] It gives no coloration with sulfuric or nitric acid. The precipitate afforded by potassium cadmium iodide solution is crystalline, mp. 118 °C, while that given by nicotine with this reagent is amorphous. Sodium nitroprusside gives a deep red colour, which disappears on warming, but reappears on cooling, and is changed to blue or violet by aldehydes.[6]
L-(R)-Coniine has [α]21°D 15° and in other respects resembles its D-isomer, but the salts have slightly different melting points; the platinichloride has mp. 160 °C (Löffler and Friedrich report 175 °C), the aurichloride mp. 59 °C.[7]
Synthesis
In the original synthesis of this substance by Ladenburg in 1886,[8] he heated methylpyridinium iodide at 250 °C to obtain 2-methylpyridine (α-picoline). 2-Methylpyridine was reacted with paraldehyde in the presence of a base to 2-propenylpyridine in a Knoevenagel condensation. This intermediate was reduced with metallic sodium in ethanol to racemic (±) coniine (reduction by hydrogen gas is also possible). Enantiopure coniine was obtained by chiral resolution — fractional crystallisation of the diastereoisomers of the salt obtained with (+)-tartaric acid.
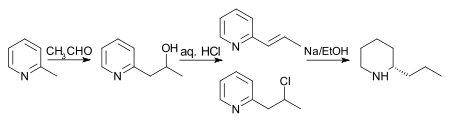
The initial reaction however gives a poor yield and was improved by interaction of the two reagents at 150 °C in sealed tubes to give methyl-2-picolylalkyne, which was then heated at 185 °C with hydrochloric acid for 10 hours, producing a mixture of 2-propenylpyridine and 2-chloropropylpyridine. This mixture was reduced to rac-coniine by sodium in ethanol.
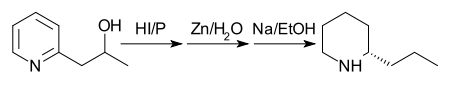
In 1907 the process was still further improved by reducing methyl-2-picolylalkine with phosphorus and hydroiodic acid at 125 °C and treating the product with zinc dust and water, then reducing the product with sodium in ethanol.[9]
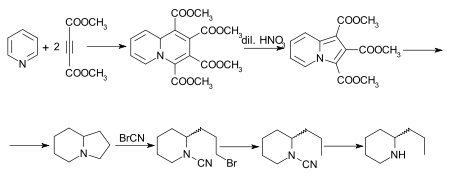
A number of other syntheses of coniine have been effected,[10] of which that of Diels and Alder is of special interest. The initial adduct of pyridine and dimethyl acetylenedicarboxylate is tetramethylquinolizine-1,2,3,4-tetracarboxylate, which on oxidation with dilute nitric acid is converted into trimethyl indolizine-tricarboxylate. This, on hydrolysis and decarboxylation, furnishes indolizine, the octahydro-derivate of which, also known as octahydropyrrocoline[11] is converted by the cyanogen bromide method successively into the bromocyanoamide, cyanoamide and rac.-coniine. A synthesis of the alkaloid, starting from indolizine (pyrrocoline) is described by Ochiai and Tsuda.[12]
The preparation of L-(R)-coniine by the reduction of β-coniceine (L-propenylpiperidine) by Löffler and Friedrich[13] is interesting as a means of passing from conhydrine to L-(R)-coniine. Hess and Eichel have shown[14] that pelletierine is the aldehyde (β-2-piperidyl-propaldehyde) corresponding to coniine, and yields rac-coniine when its hydrazone is heated with sodium ethoxide in ethanol at 156-170 °C. According to these authors, D-(S)-coniine is rendered almost optically inactive when heated with barium hydroxide and alcohol at 180-230 °C. Leithe[15] has shown by observation of the optical rotation of (+)-pipecolic acid (piperidine-2-carboxylic acid) and some of its derivatives under varying conditions,[16] that it must belong to the D-series of amino acids, and since (+)-conhydrine can be oxidised to (-)-pipecolic acid,[17] and transformed through β-coniceine into L-(R)-(-)-coniine,[18] it follows that (+)-coniine, (+)-2-methylpiperidine (α-pipecoline) and (+)-piperidine-2-carboxylic acid must all have similar spatial configurations.
Pharmacology
Coniine paralyzes muscles by blocking the nicotinic receptor on the post-synaptic membrane of the neuromuscular junction causing a flaccid paralysis. This action is similar to that of curare. Symptoms of paralysis occur within a half hour, and death may take several hours. As the central nervous system is not affected the person remains conscious and aware until respiratory paralysis results in cessation of breathing. The muscular paralysis is an ascending flaccid paralysis as the lower limbs are affected first. The person may have an hypoxic convulsion just prior to death but this is greatly disguised by the muscular paralysis and the person may just weakly shudder. The cause of death is lack of oxygen to the brain and heart as a consequence of respiratory paralysis. A poisoned person will recover if artificial ventilation (breathing) is maintained until the toxin is removed from the receptor. Historically this is the poison that killed Socrates.
There have been a number of cases of poisoning in certain regions of Italy due to the consumption of larks and chaffinches, which eat the buds of poison hemlock during April and May. Also, the alkaloid appears to have an addictive effect: goats, cows and pigs have all shown a preference for conium-containing foliage (up to the point of eventual death) if they survive initial exposure.
Coniine in Literature
Coniine is the poison used to kill Amyas Crale in Five Little Pigs (published in 1943), also known as Murder in Retrospect, one of Agatha Christie's Hercule Poirot mysteries.
References
- ^ Arch. Pharm., 1827, 20, 97.
- ^ Annalen, 1849, 70, 73.
- ^ Ber., 1881, 14, 705.
- ^ Späth, Kuffner and Ensfellner, Ber., 1933, 66, 596.
- ^ Melzer, Arch. Pharm., 1898, 236, 701; cf. Dilling, Pharm. J., 1909, [iv], 29, 34, 70, 102.
- ^ Gabutti, Chem. Soc. Abstr., 1906, [ii], 711.
- ^ Ahrens, Ber., 1902, 35, 1330; Löffler and Friedrich, ibid., 1909, 42, 107.
- ^ Chem. Soc. Abstr., 1886, 19, 439, 2579.
- ^ Ber., 1893, 26, 854; 1894, 27, 853, 859, 3063; 1896, 29, 2706; 1901, 34, 3416; 1906, 39, 2486; 1907, 40, 3734; cf. Wolffenstein, ibid., 1894, 27, 2611, 2615; 1896, 29, 1956; Simon, Bull. Soc. chim., 1894, [iii], 9, 949; Landolt, Ber., 1894, 27, 1362; Hess and Weltzien, ibid., 1920, 53, 139.
- ^ Engler and Baur, Ber., 1891, 24, 2530; 1894, 27, 1775; Lautenschlager and Onsanger, ibid., 1918, 51, 602; Koller, Monats., 1926, 47, 393; Diels and Alder, Annalen, 1932, 498, 16.
- ^ Clemo and Ramage, J. Chem. Soc., 1932, 2969.
- ^ Ber., 1934, 67, 1011.
- ^ Ber., 1909, 42, 107.
- ^ Ber., 1917, 50, 1192, 1386.
- ^ Ber., 1932, 65, 927.
- ^ Cf. Clough, J. Chem. Soc., 1918, 113, 526.
- ^ Willstätter, Ber., 1901, 34, 3166.
- ^ Löffler and Friedrich, Ber., 1909, 42, 107.
External links
Ancient anaesthesia Plants/animals Aconite • Castoreum • Cannabis • Coca • Deadly nightshade • Henbane • Lactucarium • Mandrake • Metel nut • Opium • Poison hemlock • Saussurea • Toloatzin • WillowPeople Abulcasis • Avenzoar • Avicenna • Celsus • Dioscorides • Galen • Hippocrates • Rhazes • Sabuncuoğlu • Sushrutha • Theophrastus • ZhangCompounds Aconitine • Δ9-THC • Atropine • Cocaine • Coniine • Hyoscyamine • Morphine • Salicylate • ScopolamineCategories:- Alkaloids
- Neurotoxins
- Piperidines
Wikimedia Foundation. 2010.