- Propionaldehyde
-
Propanal 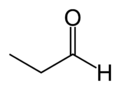

 PropanalSystematic namePropionaldehydeOther namesMethylacetaldehyde; propionic aldehyde; propaldehyde
PropanalSystematic namePropionaldehydeOther namesMethylacetaldehyde; propionic aldehyde; propaldehydeIdentifiers CAS number 123-38-6 
PubChem 527 ChemSpider 512 
UNII AMJ2B4M67V 
UN number 1275 ChEBI CHEBI:17153 
ChEMBL CHEMBL275626 
Jmol-3D images Image 1 - CCC=O
Properties Molecular formula C3H6O Molar mass 58.08 g mol−1 Appearance Colorless liquid
Pungent, marty odorDensity 0.81 g cm−3 Melting point −81 °C, 192 K, -114 °F
Boiling point 46-50 °C, 319-323 K, 115-122 °F
Solubility in water 20 g/100 mL Viscosity 0.6 cP at 20°C Structure Molecular shape C1, O: sp2 C2, C3: sp3
Dipole moment 2.52 D Hazards EU classification Highly flammable (F)
Irritant (Xi)R-phrases R11, R36/37/38 S-phrases S9, S16, S29 NFPA 704 Flash point −26 °C Autoignition
temperature175 °C Related compounds Related aldehydes Acetaldehyde
Butyraldehyde (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is a saturated 3-carbon aldehyde and is a structural isomer of acetone. It is a colourless liquid with a slightly irritating, fruity odour.
Contents
Production
Propionaldehyde is mainly produced industrially through hydroformylation, by combining synthesis gas (carbon monoxide and hydrogen) with ethylene using a metal catalyst:
- CO + H2 + C2H4 → CH3CH2CHO
Laboratory preparation
In the laboratory, it may be prepared by refluxing a mixture of propanol, sulfuric acid, and potassium dichromate. The reflux condenser contains water heated at 60 °C, which condenses unreacted propanol, but allows propionaldehyde to pass. The propionaldehyde vapor is immediately condensed into a suitable receiver. In this arrangement, any propionaldehyde formed is immediately removed from the reactor, thus it does not get over-oxidized to propionic acid.[1]
Uses
It is principally used as a precursor to trimethylolethane (CH3C(CH2OH)3) through a condensation reaction with methanol; this triol is an important intermediate in the production of alkyd resins.
Condensation of propionaldehyde with tert-butylamine gives CH3CH2CH=N-t-Bu, a three-carbon building block used in organic synthesis. Deprotonation of this imine with LDA produces CH3CHLiCH=N-t-Bu, which in turn condenses with aldehydes.[2]
Recent Interstellar Discoveries
From 2004 on, researchers have discovered new interstellar molecules one of which is propanal. The team detected acetamide, cyclopropenone, propenal, propanal and ketenimine in Sagittarius B2 (N), while methyl-cyano-diacetylene, methyl-triacetylene and cyanoallene were found in the Taurus Molecular Cloud (TMC-1). Sagittarius B2 (N) is near the center of the Milky Way Galaxy, about 26,000 light years from Earth, and the Taurus Molecular Cloud is about 450 light years from Sagittarius B2 (N). Two years of work led to the molecules' discoveries, "a feat unprecedented in the 35-year history of searching for complex molecules in space and suggests that a universal prebiotic chemistry is at work", said Jan M. Hollis of NASA's Goddard Space Flight Center in Greenbelt, Md., the research team's leader. For the team to identify the propanal they looked for the emission of specific frequencies of radio waves from the cloud. They did this because different types of molecules emit energy at different frequencies, each producing a unique signal that researchers can detect with powerful telescopes. Located in the July 20th Astrophysical Journal Letters, researchers reported that they had recorded the frequencies associated with two aldehydes called propenal and propanal. Although researchers have found other organic molecules in space before this, the evidence of the two aldehydes will assist them in the understanding of how molecular building blocks are assembled into more-complex organic molecules in space.
References
- ^ Charles D. Hurd and R. N. Meinert (1943), "Propionaldehyde", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2p0541; Coll. Vol. 2: 541
- ^ Peralta, M. M. "Propionaldehyde t-Butylimine" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. DOI: 10.1002/047084289.
- Chemical Precursors to Life Found in Space Scientists say that a universal prebiotic chemistry may be at work
- Two newly found space molecules. By: Goho, Alexandra, Science News, 00368423, 7/24/2004, Vol. 166, Issue 4
Categories:- Aldehydes
- Hazardous air pollutants
Wikimedia Foundation. 2010.

