- Meldrum's acid
-
Meldrum's Acid  2,2-Dimethyl-1,3-dioxane-4,6-dione
2,2-Dimethyl-1,3-dioxane-4,6-dioneIdentifiers CAS number 2033-24-1 PubChem 16249 Jmol-3D images Image 1 - O=C1OC(OC(=O)C1)(C)C
- InChI=1/C6H8O4/c1-6(2)9-4(7)3-5(8)10-6/h3H2,1-2H3
InChI Key - GXHFUVWIGNLZSC-UHFFFAOYAM (Std. InChIKey)
Properties Molecular formula C6H8O4 Molar mass 144.13 g mol−1 Melting point 94-95 °C (decomposes)[1]
Acidity (pKa) 4.97  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
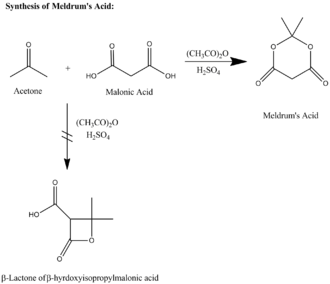
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound. The compound was first made in 1908 by Andrew Norman Meldrum by a condensation reaction of malonic acid with acetone in acetic anhydride and sulfuric acid.[2] Meldrum misidentified the structure as a β-lactone of β-hydroxyisopropylmalonic acid. The correct structure[3] is shown on this page.
As an alternative to its original preparation, Meldrum's acid can be synthesized from malonic acid, isopropenyl acetate, and catalytic sulfuric acid. Meldrum's acid has a high acidity with a pKa of 4.97. Meldrum's acid high acidity was long considered "anomalous" given it is 8 orders of magnitude more acidic than the highly related compound, dimethyl malonate (whose aqueous pKa is around 13). In 2004, Ohwada and coworkers resolved the Meldrum acid anomaly by performing several calculations.[4] Ohwada noticed that the energy-minimizing conformation structure of the compound places the alpha proton's σCH orbital in the proper geometry to align with the π*CO, so that the ground state poses unusually strong destabilization of the C-H bond.
Because of its great acidity, Meldrum's acid, like malonic acid, can serve as a reactant in Knoevenagel condensations.
Contents
Ketene formation
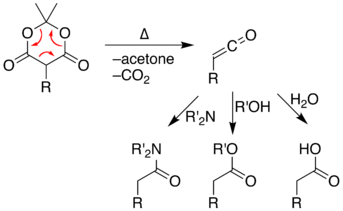
Meldrum's acid displays thermal instability. At high temperatures, Meldrum's acid undergoes a pericyclic reaction that releases acetone and carbon dioxide and produces a highly reactive ketene compound.[5] Ketene intermediates can be isolated using flash vacuum pyrolysis (FVP) of Meldrum's acid at high temperatures. These highly electrophilic ketenes allow various chemical reactions to take place by reaction the unstable ketene with other chemicals. Alternately, the pyrolysis can be performed in solution, allowing a one-pot reaction with chemicals already present rather than isolating the unstable ketene intermediate. With relative simplicity, is possible to form new C–C bonds, rings, amides, esters, and acids. The ability to form such diverse products makes Meldrum's acid a very useful reagent for synthetic chemists.[6]
Alkylation and acylation
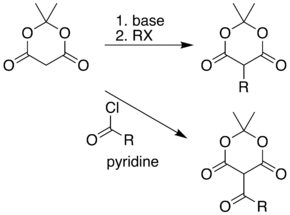
The position between the two carbonyl groups in Meldrum's acid is acidic, allowing simple alkylation and acylation at this position. For example, deprotonation and reaction with a simple alkyl halide allows formation of products containing carbon chains attached to the ring. Alternatively, this position can be acylated with a carboxylic acid chloride (RCOCl).
These two reactions allow Meldrum's acid to serve as a starting scaffold for the synthesis of many different structures with various functional groups. Paired with the pyrolysis and nucleophilic trapping reaction, above, the alkylated products can be carried on to produce a range of different amide and ester compounds. Heating the acyl product in the presence of an alcohol leads to ester exchange and decarboxylation in a process similar to the malonic ester synthesis. The reactive nature of the cyclic-diester allows good reactivity even for alcohols as hindered as t-butanol.[7] Ketoesters of this type are useful in the Knorr pyrrole synthesis.
References
- ^ "Meldrum's Acid". The Merck Index. 14th. edition. Merck Research Laboratories. 2006. pp. 1005. ISBN 978-0-911910-00-1.
- ^ Meldrum, Andrew Norman (1908). "A β-lactonic acid from acetone and malonic acid". Journal of the Chemical Society, Transactions 93: 598–601. doi:10.1039/CT9089300598. http://books.google.com/?id=PMM3AAAAMAAJ&pg=PA598&lpg=PA598&dq=andrew+norman+meldrum.
- ^ Davidson, David; Bernhard, Sidney A. (1948). "The Structure of Meldrum's Supposed β-Lactonic Acid". Journal of the American Chemical Society 70 (10): 3426–3428. doi:10.1021/ja01190a060. PMID 18891879.
- ^ Nakamura, Satoshi; Hirao, Hajime; Ohwada, Tomohiko (2004). "Rationale for the Acidity of Meldrum's Acid. Consistent Relation of C−H Acidities to the Properties of Localized Reactive Orbital". Journal of Organic Chemistry 69 (13): 4309–4316. doi:10.1021/jo049456f. PMID 15202884.
- ^ Dumas, Aaron M./Fillion, Eric. (2009)Meldrum's Acids and 5-Alkylidene Meldrum's Acids in Catalytic Carbon-Carbon Bond-Forming Processes. Accounts of Chemical Research. 43 (3): 440-454. doi=10.1021/ar900229z
- ^ Lipson, Victoria V./Gorobets, Nikolay Yu. (2009)One hundred years of Meldrum's acid: advances in the synthesis of pyridine and pyrimidine derivatives. Mol Divers. 13: 399-419.
- ^ Oikawa, Yuji; Sugano, Kiyoshi; Yonemitsu, Osamu (1978). "Meldrum's acid in organic synthesis. 2. A general and versatile synthesis of β-keto esters". Journal of Organic Chemistry 43 (10): 2087–2088. doi:10.1021/jo00404a066.
Further reading
- Kidd, Hamish (November 2008). "Meldrum's Acid". Chemistry World: 35–36.
- Lipson VV & Gorobets NY (2009). "One hundred years of Meldrum’s acid: advances in the synthesis of pyridine and pyrimidine derivatives". Molecular Diversity 13 (4): 339–419. doi:10.1007/s11030-009-9136-x. PMID 19381852.
- McNab, Hamish (1978). "Meldrum's Acid". Chemical Society Reviews 7 (3): 345–358. doi:10.1039/CS9780700345.
External links
- Synlett Spotlight Website
- Meldrum's acid in Organic Syntheses Website
Categories:- Organic acids
- Lactones
- Dioxanes
Wikimedia Foundation. 2010.