- I-cell disease
-
I-cell disease Classification and external resources 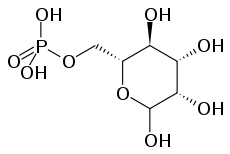
Mannose-6-phosphate (M6P). I-cell disease involves a failure to add M6P to proteins.ICD-10 E77.0 ICD-9 272.7 OMIM 252500 DiseasesDB 29175 eMedicine ped/1150 MeSH D009081 Inclusion-cell (I-cell) disease, also referred to as mucolipidosis II (ML II),[1][2] is part of the lysosomal storage disease family and results from a defective phosphotransferase (an enzyme of the Golgi apparatus). This enzyme transfers phosphate to mannose residues on specific proteins, and serves as a marker for them to be targeted to lysozomes within the cell. Without this marker, the proteins are instead excreted outside the cell -- the default pathway for proteins moving through the Golgi apparatus. Lysozomes cannot function without these proteins, which function as catabolic enzymes for the normal breakdown of substances throughout the body. As a result, a build up of these substances occurs within lysosomes because they cannot be degraded, resulting in the characteristic "I cells," or "inclusion cells." These cells can be identified under the microscope. In addition, the defective lysozomal enzymes normally found only within lysozomes are instead found in high concentrations in the blood.
Contents
Presentation
ML II is a particularly severe form of ML that resembles one of the mucopolysaccharidoses called Hurler syndrome. Typically, by the age of 6 months, failure to thrive and developmental delays are obvious symptoms of this disorder. Some physical signs, such as abnormal skeletal development, coarse facial features, and restricted joint movement, may be present at birth. Children with ML II usually have enlargement of certain organs, such as the liver (hepatomegaly) or spleen (splenomegaly), and sometimes even the heart valves. Affected children often have stiff claw-shaped hands and fail to grow and develop in the first months of life. Delays in the development of their motor skills are usually more pronounced than delays in their cognitive (mental processing) skills. Children with ML II eventually develop a clouding on the cornea of their eyes and, because of their lack of growth, develop short-trunk dwarfism (underdeveloped trunk). These young patients are often plagued by recurrent respiratory tract infections, including pneumonia, otitis media (middle ear infections), bronchitis and carpal tunnel syndrome. Children with ML II generally die before their seventh year of life, often as a result of congestive heart failure or recurrent respiratory tract infections.
Pathophysiology
I-cell disease is an autosomal recessive disorder caused by a deficiency of GlcNAc phosphotransferase, which phosphorylates mannose residues to mannose-6-phosphate on N-linked glycoproteins in the Golgi apparatus within the cell. Without mannose-6-phosphate to target them to the lysosomes, the enzymes are transported from the Golgi to the extracellular space, resulting in large intracellular inclusions of molecules requiring lysosomal degradation in patients with the disease (hence the name of the disorder).[3] Hydrolases secreted into the blood stream cause little problem as they are deactivated in the neutral pH of the blood.
It can be associated with GNPTA.[4] In a case report, it was complicated by severe dilative cardiomyopathy(DCM)[5]
Treatment
There is no cure yet for I-Cell disease/Mucolipidosis II disease. Treatment is limited to controlling or reducing the symptoms that are associated with this disorder. Nutrition supplements, particularly iron and vitamin B12, are often recommended for individuals with I-Cell disease. Physical therapy to improve motor delays and speech therapy to improve language acquisition are treatment options. Surgery can remove the thin layer of corneal clouding to temporarily improve the complication. It is possible that bone marrow transplant may be helpful in delaying or correcting the neurological deterioration that occurs with I-Cell disease.[1]
See also
References
- ^ "mucolipidosis II" at Dorland's Medical Dictionary
- ^ Plante M, Claveau S, Lepage P et al. (March 2008). "Mucolipidosis II: a single causal mutation in the N-acetylglucosamine-1-phosphotransferase gene (GNPTAB) in a French Canadian founder population". Clin. Genet. 73 (3): 236–44. doi:10.1111/j.1399-0004.2007.00954.x. PMID 18190596. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0009-9163&date=2008&volume=73&issue=3&spage=236.
- ^ Champe, Pamela (2004). Lippincott's Illutrated Reviews: Biochemistry. Richard A Harvey, Denise R Ferrier (3rd ed.). Philadelphia, Pa.: Lippincott-Raven. p. 167. ISBN 0781722659.
- ^ Tiede S, Storch S, Lübke T et al. (2005). "Mucolipidosis II is caused by mutations in GNPTA encoding the alpha/beta GlcNAc-1-phosphotransferase". Nat. Med. 11 (10): 1109–12. doi:10.1038/nm1305. PMID 16200072.
- ^ http://www.sahha.gov.mt/pages.aspx?page=752
External links
- mucolipidoses at NINDS - article derived from detail sheet available here
- I cell disease at NIH's Office of Rare Diseases
- GeneReview/NIH/UW entry on Mucolipidosis II
- Hide and Seek Foundation For Lysosomal Disease Research
(LSD) Inborn error of carbohydrate metabolism: glycoproteinosis (E77, 271.8) Anabolism Post-translational modification
of lysosomal enzymesCatabolism Aspartylglucosaminuria · Fucosidosis · mannosidosis (Alpha-mannosidosis, Beta-mannosidosis) · Sialidosis · Schindler diseaseOther Translation Ribosome: Diamond–Blackfan anemia · FMR1 (Fragile X syndrome, Fragile X-associated tremor/ataxia syndrome, Premature ovarian failure 1)
Initiation factor: Leukoencephalopathy with vanishing white matter
snRNP: Retinitis pigmentosa 33Posttranslational modification I-cell diseaseE1: X-linked spinal muscular atrophy 2
E3: Johanson–Blizzard syndrome · Von Hippel–Lindau disease · 3-M syndrome · Angelman syndrome
Deubiquitinating enzyme: Machado–Joseph disease · Aneurysmal bone cyst · Multiple familial trichoepithelioma 1Othersee also genetic translation, posttranslational modification
B structural (perx, skel, cili, mito, nucl, sclr) · DNA/RNA/protein synthesis (drep, trfc, tscr, tltn) · membrane (icha, slcr, atpa, abct, othr) · transduction (iter, csrc, itra), trfkCategories:- Glycoprotein metabolism disorders
Wikimedia Foundation. 2010.
