- Deubiquitinating enzyme
-
Deubiquitinating enzymes (DUBs) are a large group of proteases[1] (more than 60 known) that regulate ubiquitin-dependent metabolic pathways by cleaving ubiquitin-protein bonds. DUBs are also commonly referred to as deubiquitinating peptidases, deubiquitinating isopeptidases, deubiquitinases, ubiquitin proteases, ubiquitin hydrolyases, ubiquitin isopeptidases, or DUbs. The human genome encodes nearly 100 DUBs with specificity for ubiquitin in five gene families.[2] Potentially, DUBs may act as negative and positive regulators of the ubiquitin system. In addition to ubiquitin recycling, they are involved in processing of ubiquitin precursors, in proofreading of protein ubiquitination and in disassembly of inhibitory ubiquitin chains.
They may be associated with disease.[3]
Contents
Classes
DUBs can be classified into two main classes: cysteine proteases and metalloproteases.
Cysteine proteases
There are four main superfamilies of cysteine protease DUBs:
- the ubiquitin-specific processing protease (USP/UBP) superfamily; (USP1, USP2, USP3, USP4, USP5, USP6, USP7, USP8, USP9X, USP9Y, USP10, USP11, USP12, USP13, USP14, USP15, USP16, USP17, USP17L2, USP17L3, USP17L4, USP17L5, USP17L7, USP17L8, USP18, USP19, USP20, USP21, USP22, USP23, USP24, USP25, USP26, USP27X, USP28, USP29, USP30, USP31, USP32, USP33, USP34, USP35, USP36, USP37, USP38, USP39, USP40, USP41, USP42, USP43, USP44, USP45, USP46)
- the ubiquitin C-terminal hydrolyase (UCH) superfamily; (UCHH2, UCHL1, UCHL3)
- the ovarian tumour (OTU) superfamily;
- and the Machado-Josephin domain (MJD) superfamily. (OTUB1, OTUB2, ATXN3, ATXN3L)
However, there is also a little known putative group of DUBs called the permutated papain fold peptidases of dsDNA viruses and eukaryote (PPPDEs) superfamily, which, if shown to be bona fide DUBs, would be the fifth in the cysteine protease class.[4]
Metalloproteases
The Jab1/Mov34/Mpr1 Pad1 N-terminal+ (MPN+) (JAMM) domain superfamily proteins bind zinc and hence are metalloproteases.
Role in the ubiquitin pathway
DUBs play several roles in the ubiquitin pathway. First, DUBs carry out activation of the ubiquitin proproteins, probably cotranslationally. Second, DUBs recycle ubiquitin that may have been accidentally trapped by the reaction of small cellular nucleophiles with the thiol ester intermediates involved in the ubiquitination of proteins. Third, DUBs reverse the ubiquitination or ubiquitin-like modification of target proteins. Fourth, DUBs are also responsible for the regeneration of monoubiquitin from unanchored polyubiquitin, i.e., free polyubiquitin that is synthesized de novo by the conjugating machinery or that has been released from target proteins by other DUBs.[2] Finally, the deubiquitinating enzymes UCH-L3 and YUH1 are able to hydrolyse mutant ubiquitin UBB+1 despite of the fact that the glycine at position 76 is mutated.[5]
Domain architecture
DUSP domain 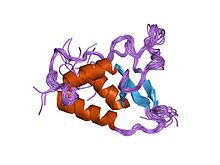
solution structure of the dusp domain of husp15 Identifiers Symbol DUSP Pfam PF06337 InterPro IPR006615 MEROPS C19 Available protein structures: Pfam structures PDB RCSB PDB; PDBe PDBsum structure summary All DUBs contain a catalytic domain surrounded by one or more subdomains, some of which contribute to target recognition. The ~120-residue DUSP (domain present in ubiquitin-specific proteases) domain is one of these specific subdomains. Single or tandem DUSP domains are located both N- and C-terminal to the ubiquitin carboxyl-terminal hydrolase catalytic core domain.[6] The DUSP domain displays a tripod-like AB3 fold with a three-helix bundle and a three-stranded anti-parallel beta-sheet resembling the legs and seat of the tripod. Conserved residues are predominantly involved in hydrophobic packing interactions within the three alpha-helices. The most conserved DUSP residues, forming the PGPI motif, are flanked by two long loops that vary both in length and sequence. The PGPI motif packs against the three-helix bundle and is highly ordered.[6] The function of the DUSP domain is unknown but it may play a role in protein/protein interaction or substrate recognition.
References
- ^ Wilkinson K (1997). "Regulation of ubiquitin-dependent processes by deubiquitinating enzymes". FASEB J. 11 (14): 1245–56. PMID 9409543.
- ^ a b Reyes Turcu FE, Ventii KH, Wilkinson KD (2009). "Activity and Cellular Roles of Ubiquitin-Specific Deubiquitinating Enzymes". Annual Review of Biochemistry 78: 363–97. doi:10.1146/annurev.biochem.78.082307.091526. PMC 2734102. PMID 19489724. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2734102.
- ^ Singhal S, Taylor MC, Baker RT (2008). "Deubiquitylating enzymes and disease". BMC Biochem. 9 Suppl 1: S3. doi:10.1186/1471-2091-9-S1-S3. PMC 2582804. PMID 19007433. http://www.biomedcentral.com/1471-2091/9%20Suppl%201/S3.
- ^ Iyer LM, Koonin EV, Aravind L (2004). "Novel predicted peptidases with a potential role in the ubiquitin signaling system". Cell Cycle 3 (11): 1440–50. doi:10.4161/cc.3.11.1206. PMID 15483401.
- ^ Dennissen, F; Kholod N, Hermes DJ, Kemmerling N, Steinbusch HW, Dantuma NP, van Leeuwen FW. (6 July). "Mutant ubiquitin (UBB(+1)) associated with neurodegenerative disorders is hydrolyzed by ubiquitin C-terminal hydrolase L3 (UCH-L3).". FEBSletters. doi:10.1016/j.febslet.2011.06.037. PMID 21762696. http://www.sciencedirect.com/science/article/pii/S0014579311005138.
- ^ a b de Jong RN, Ab E, Diercks T, Truffault V, Daniels M, Kaptein R, Folkers GE (February 2006). "Solution structure of the human ubiquitin-specific protease 15 DUSP domain". J. Biol. Chem. 281 (8): 5026–31. doi:10.1074/jbc.M510993200. PMID 16298993.
Chaperones/
protein foldingOtherProtein targeting Ubiquitin E1 Ubiquitin-activating enzyme (UBA1, UBA2, UBA3, UBA5, UBA6, UBA7, ATG7, NAE1, SAE1)
E2 Ubiquitin-conjugating enzyme (A • B • C • D1, D2, D3 • E1, E2, E3 • G1, G2 • H • I • J1, J2 • K • L1, L2, L3, L4, L6 • M • N • O • Q1, Q2 • R1 (CDC34), R2 • S • V1, V2 • Z)
E3 Ubiquitin ligase (VHL, Cullin, CBL, MDM2, FANCL, UBR1)
Deubiquitinating enzyme: Ataxin 3 • USP6 • CYLD
ATG3 • BIRC6 • UFC1Other see also posttranslational modification disorders
B bsyn: dna (repl, cycl, reco, repr) · tscr (fact, tcrg, nucl, rnat, rept, ptts) · tltn (risu, pttl, nexn) · dnab, rnab/runp · stru (domn, 1°, 2°, 3°, 4°)This article includes text from the public domain Pfam and InterPro IPR006615
Categories:- Protein domains
- Enzymes
Wikimedia Foundation. 2010.
