- Oocyte
-
Oocyte 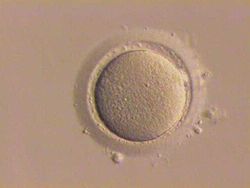
"Stripped" human oocyte; granulosa cells that had surrounded this oocyte have been removed. Gray's subject #3 38 MeSH Oocytes An oocyte, ovocyte, or rarely ocyte, is a female gametocyte or germ cell involved in reproduction. In other words, it is an immature ovum, or egg cell. An oocyte is produced in the ovary during female gametogenesis. The female germ cells produce a primordial germ cell (PGC) which undergoes a mitotic division to form an oogonium. During oogenesis the oogonium becomes a primary oocyte (pronounced oh'a-site).
Contents
Formation
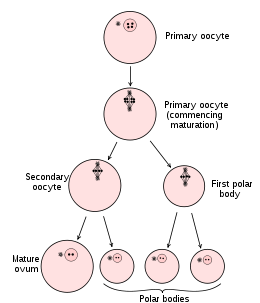 Diagram showing the reduction in number of the chromosomes in the process of maturation of the ovum.
Diagram showing the reduction in number of the chromosomes in the process of maturation of the ovum.
The formation of an oocyte is called oocytogenesis, which is a part of oogenesis.[1] Oogenesis results in the formation of both primary oocytes before birth, and of secondary oocytes after it as part of ovulation.
Cell type ploidy/chromosomes chromatids Process Time of completion Oogonium diploid/46 2N Oocytogenesis (mitosis) third trimester primary Oocyte diploid/46 2N Ootidogenesis (meiosis 1) (Folliculogenesis) Dictyate in prophase I until ovulation secondary Oocyte haploid/23 1N Ootidogenesis (meiosis 2) Halted in metaphase II until fertilization Ootid haploid/23 1N Ootidogenesis (meiosis 3) Minutes after fertilization Ovum haploid/23 1N Characteristics
Cytoplasm
Oocytes are rich in cytoplasm which contains yolk granules to nourish the cell early in development.
Nucleus
During the primary oocyte stage of oogenesis, the nucleus is called a germinal vesicle.[2]
The only normal human type of secondary oocyte has the 23rd (sex) chromosome as 23,X (female-determining), whereas sperm can can have 23,X (female-determining) or 23,Y (male-determining).
Nest
The space wherein an ovum or immature ovum is located is the cell-nest.[3]
Maternal Contributions to the Oocyte
Because the fate of an oocyte is to become fertilized and ultimately grow into a fully functioning organism, it must be ready to regulate multiple cellular and developmental processes. The oocyte, a large and complex cell, must be supplied with numerous molecules that will direct the growth of the embryo and control cellular activities. As the oocyte is a product of female gametogenesis, the maternal contribution to the oocyte and consequently the newly fertilized egg is enormous. There are many types of molecules that are maternally supplied to the oocyte which will direct various activities within the growing zygote.
Maternal mRNAs and Proteins
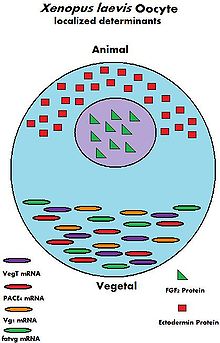
During the growth of the oocyte, a variety of maternally transcribed messenger RNAs, or mRNAs, are supplied by maternal cells. These mRNAs can be stored in mRNP (message ribonucleoprotein) complexes and be translated at specific time points, they can be localized within a specific region of the cytoplasm, or they can be homogeneously dispersed within the cytoplasm of the entire oocyte.[4] Maternally loaded proteins can also be localized or ubiquitous throughout the cytoplasm. The translated products of the mRNAs and the loaded proteins have multiple functions; from regulation of cellular "house-keeping" such as cell cycle progression and cellular metabolism, to regulation of developmental processes such as fertilization, activation of zygotic transcription, and formation of body axes.[4] Below are some examples of maternally inherited mRNAs and proteins found in Xenopus laevis oocytes.
Name Type of Maternal Molecule Localization Function VegT[5] mRNA Vegetal Hemisphere Transcription Factor Vg1[6] mRNA Vegetal Hemisphere Transcription Factor XXBP-1[7] mRNA Not Known Transcription Factor CREB[8] Protein Ubiquitous Transcription Factor FoxH1[9] mRNA Ubiquitous Transcription Factor p53[10] Protein Ubiquitous Transcription Factor Lef/Tcf[11] mRNA Ubiquitous Transcription Factor FGF2[12] Protein Nucleus Not Known FGF2, 4, 9 FGFR1[11] mRNA Not Known FGF Signaling Ectodermin[13] Protein Animal Hemisphere Ubiquitin Ligase PACE4[14] mRNA Vegetal Hemisphere Proprotein Convertase Coco[15] Protein Not Known BMP inhibitor Twisted Gastrulation[11] Protein Not Known BMP/Chordin Bindng Protein fatvg[16] mRNA Vegetal Hemisphere Germ Cell Formation and Cortical Rotation Maternal Mitochondria
The oocyte receives mitochondria from maternal cells, which will go on to control embryonic metabolism and apoptotic events.[4] The partitioning of mitochondria is carried out by a system of microtubules which will localize mitochondria throughout the oocyte. In certain organisms, such as mammals, paternal mitochondria brought to the oocyte by the spermatozoon are degraded through the attachment of ubiquitinated proteins. The destruction of paternal mitochondria ensures the strictly maternal inheritance of mitochondria and mitochondrial DNA or mtDNA.[4]
Maternal Nucleolus
In mammals, the nucleolus of the oocyte is derived solely from maternal cells.[17] The nucleolus, a structure found within the nucleus, is the location where rRNA is transcribed and assembled into ribosomes. While the nucleolus is dense and inactive in a mature oocyte, it is required for proper development of the embryo.[17]
Maternal Ribosomes
Maternal cells also synthesize and contribute a store of ribosomes that are required for the translation of proteins before the zygotic genome is activated. In mammalian oocytes, maternally derived ribosomes and some mRNAs are stored in a structure called cytoplasmic lattices. These cytoplasmic lattices, a network of fibrils, protein, and RNAs, have been observed to increase in density as the number of ribosomes decrease within a growing oocyte.[18]
Paternal Contributions to the Oocyte
The spermatozoon which fertilizes an oocyte will contribute its pronucleus, the other half of the zygotic genome. In some species, the spermatozoon will also contribute a centriole which will help make up the zygotic centrosome required for the first division. However, in some species, such as in the mouse, the entire centrosome is acquired maternally.[19] Currently under investigation is the possibility of other cytoplasmic contributions made to the embryo by the spermatozoon.
Abnormalities
- nondisjunction -- a failure of proper homolog separation in meiosis I, or sister chromatid separation in meiosis II can lead to aneuploidy, in which the oocyte has the wrong number of chromosomes, for example 22,X or 24,X. This is the cause of conditions like Down syndrome and Edwards syndrome. It is more likely with advanced maternal age.
- Some oocytes have multiple nuclei, although it is thought they never mature.
References
- ^ answers.com
- ^ Biology-online
- ^ Grier HJ, Uribe MC, Parenti LR (April 2007). "Germinal epithelium, folliculogenesis, and postovulatory follicles in ovaries of rainbow trout, Oncorhynchus mykiss (Walbaum, 1792) (Teleostei, protacanthopterygii, salmoniformes)". J. Morphol. 268 (4): 293–310. doi:10.1002/jmor.10518. PMID 17309079.
- ^ a b c d Mtango N.R., Potireddy S., Latham K.E. (2008). Oocyte quality and maternal control of development. Int. Rev. Cell Mol. Biol. 268, 223-290.
- ^ Zhang J., King M.L. (1996). Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development. 12, 4119–29.
- ^ Heasman J., Wessely O., Langland R., Craig E.J., Kessler D.S. (2001). Vegetal localization of maternal mRNAs is disrupted by VegT depletion. Dev Biol. 240, 377–386.
- ^ Zhao H., Cao Y., Grunz H. (2003). Xenopus X-box binding protein 1, a leucine zipper transcription factor, is involved in the BMP signaling pathway. Dev Biol. 257, 278–291.
- ^ Sundaram N., Tao Q., Wylie C., Heasman J. (2003). The role of maternal CREB in early embryogenesis of Xenopus laevis. Dev Biol. 261, 337–352.
- ^ Kofron M., Puck H., Standley H., Wylie C., Old R., Whitman M., et al. (2004). New roles for FoxH1 in patterning the early embryo. Development. 131, 5065–5078.
- ^ Takebayashi-Suzuki K., Funami J., Tokumori D., Saito A., Watabe T., Miyazono K., et al. (2003). Interplay between the tumor suppressor p53 and TGF beta signaling shapes embryonic body axes in Xenopus. Development. 130, 3929–3939.
- ^ a b c Heasman, J. (2006). Maternal determinants of embryonic cell fate. Semin. Cell Dev. Biol. 17, 93-98.
- ^ Song J., Slack J.M. (1994). Spatial and temporal expression of basic fibroblast growth factor (FGF-2) mRNA and protein in early Xenopus development. Mech Dev. 48, 141–151.
- ^ Dupont S., Zacchigna L., Cordenonsi M., Soligo S., Adorno M., Rugge M., et al. (2005). Germ-layer specification and control of cell growth by Ectodermin, a Smad4 ubiquitin ligase. Cell. 121, 87–99.
- ^ Birsoy B., Berg L., Williams P.H., Smith J.C., Wylie C.C., Christian J.L., et al. (2005). XPACE4 is a localized pro-protein convertase required for mesoderm induction and the cleavage of specific TGFbeta proteins in Xenopus development. Development. 132, 591–602.
- ^ Bell E., Munoz-Sanjuan I., Altmann C.R., Vonica A., Brivanlou A.H. (2003). Cell fate specification and competence by Coco, a maternal BMP, TGFbeta and Wnt inhibitor. Development. 130, 1381–1389.
- ^ Chan A. P., Kloc M., Larabell C. A., LeGros M., Etkin L.D. (2007). The maternally localized RNA fatvg is required for cortical rotation and germ cell formation. Mech Dev. 124, 350-363.
- ^ a b Ogushi S., et al. (2008). The maternal nucleolus is essential for early embryonic development in mammals. Science. 319, 613-616
- ^ Vitale A.M., Yurttas P., Fitzhenry R.J., Cohen-Gould, L., Wu W., Gossen J.A., Coonrod S.A. (2009). Role for PADI6 and the CPLs in ribosomal storage in oocytes and translation in the early embryo. Development. 135, 2627-2636.
- ^ Sutovsky P., Schatten G. (2000). Paternal contributions to the mammalian zygote: fertilization after sperm-egg fusion. Int. Rev. Cytol. 195, 1-65.
Resources
William K. Purves, Gordon H. Orians, David Sadava, H. Craig Heller, Craig Heller (2003). Life: The Science of Biology(7th ed.), pp. 823–824
See also
External links
Preceded by
noneStages of human development
Sperm cell + OocyteSucceeded by
ZygoteCategories:- Germ cells
Wikimedia Foundation. 2010.