- Phenacyl chloride
-
Phenacyl chloride 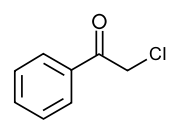
 2-chloro-1-phenylethanone
2-chloro-1-phenylethanoneIdentifiers CAS number 532-27-4 
PubChem 10757 ChemSpider 10303 
ChEMBL CHEMBL105712 
Jmol-3D images Image 1 - c1ccc(cc1)C(=O)CCl
- InChI=InChI=1S/C8H7ClO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6H2
Properties Molecular formula C8H7ClO Molar mass 154.59 g mol−1 Melting point 54-56 °C, 327-329 K, 129-133 °F
Hazards MSDS Oxford MSDS EU classification  T
T chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Phenacyl chloride is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN.
Preparation
Phenacyl chloride is readily available commercially. It may be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst:[1]
Riot control agent
It was investigated, but not used, during the First and Second World Wars. Because of its greater toxicity, it has largely been supplanted by CS gas.
CN is still supplied to paramilitary and police forces in a small pressurized aerosol can known as “Mace” or tear gas. Its use has fallen by the wayside as pepper spray both works and disperses more quickly than CN.
The term "Mace" came into being because it was the brand-name invented by one of the first American manufacturers of CN aerosol sprays. Subsequently, Mace became synonymous with tear-gas sprays in the same way that Kleenex has become strongly associated with tissue papers (a phenomenon known as a genericized trademark).
Like CS gas, this compound irritates the mucous membranes (oral, nasal, conjunctival and tracheobronchial). Sometimes it can give rise to more generalized reactions such as syncope, temporary loss of balance and orientation. More rarely, cutaneous irritating outbreaks have been observed and allergic contact permanent dermatitis.
At high concentrations CN has caused corneal epithelial damage and chemosis. It has also accounted for at least five deaths, which have resulted from pulmonary injury and/or asphyxia.[2]
References
- ^ Nathan Levin and Walter H. Hartung (1955), "ω-Chloroisonitrosoacetophenone", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0191; Coll. Vol. 3: 191
- ^ Blain, P. G. (2003). "Tear gases and irritant incapacitants. 1-chloroacetophenone, 2-chlorobenzylidene malononitrile and dibenz[b,f]-1,4-oxazepine". Toxicol Rev 22 (2): 103–10. PMID 15071820. http://www.ingentaconnect.com/content/adis/txr/2003/00000022/00000002/art00005.
Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control 
This article related to weaponry is a stub. You can help Wikipedia by expanding it.

