- Diphosgene
-
Diphosgene 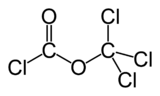
 DiphosgeneOther namestrichloromethyl chloroformate
DiphosgeneOther namestrichloromethyl chloroformateIdentifiers CAS number 503-38-8 RTECS number LQ7350000 Properties Molecular formula C2Cl4O2 Molar mass 197.82 g/mol Appearance liquid at room temperature Density 1.65 g/cm3 Melting point -57 °C
Boiling point 128 °C
Solubility in water insol. Hazards R-phrases 26/28-34 S-phrases 26-28-36/37/39-45 Main hazards toxic Related compounds Related compounds COCl2, Cl2  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Diphosgene is a chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene but is more conveniently handled because it is a liquid, whereas phosgene is a gas.
Contents
Production and uses
Diphosgene is prepared by radical chlorination of methyl chloroformate under UV light.[1]:
- Cl-CO-OCH3 + 3 Cl2 —(hv)→ Cl-CO-OCCl3 + 3 HCl
Another method is the radical chlorination of methyl formate[2]:
- H-CO-OCH3 + 4 Cl2 —(hv)→ Cl-CO-OCCl3 + 4 HCl
Diphosgene converts to phosgene upon heating or upon catalysis with charcoal. It is thus useful for reactions traditionally relying on phosgene. For example, it convert amines into isocyanates, secondary amines into carbamoyl chlorides, carboxylic acids into acid chlorides, and formamides into isocyanides. Diphosgene serves as a source of two equivalents of phosgene:
- 2 RNH2 + ClCO2CCl3 → 2 RNCO + 4 HCl
With α-amino acids diphosgene gives the acid chloride-isocyanates, OCNCHRCOCl, or N-carboxy-amino acid anhydrides depending on the conditions.[3]
It hydrolyzes to release HCl in humid air.
Diphosgene has supplanted phosgene in some large scale industrial reactions such as the production of (di-)isocyanates from amines because it is safer to handle than phosgene.
Role in warfare
Diphosgene was originally developed as a pulmonary agent for chemical warfare, a few months after the first use of phosgene. It was used as a poison gas in artillery shells by Germany during World War I. The first recorded battlefield use was in May 1916.[4] Diphosgene was developed because the vapors could destroy the filters in gas masks in use at the time.
Safety
Diphosgene has a relatively high vapor pressure of 10 mmHg (1.3 kPa) at 20 °C and decomposes to phosgene around 300 °C. Exposure to diphosgene is similar in hazard to phosgene and the MSDS should be consulted.
See also
References
- ^ Keisuke Kurita1 and Yoshio Iwakura (1979), "TRICHLOROMETHYL CHLOROFORMATE AS A PHOSGENE EQUIVALENT: 3-ISOCYANATOPROPANOYL CHLORIDE", Org. Synth. 59: 195, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0715; Coll. Vol. 6: 715
- ^ Lohs, KH.: Synthetische Gifte; Berlin (east), 1974 (german)
- ^ Kurita, K. "Trichloromethyl Chloroformate" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. DOI: 10.1002/047084289.
- ^ Jones, Simon; Hook, Richard (2007). World War I Gas Warfare Tactics and Equipment. Osprey Publishing. ISBN 1846031516.
External links
- medical care guide.
- NATO guide, includes treatment advice
- material safety data sheet (PDF, for phosgene and diphosgene treated as one).
Blood Blister Ethyldichloroarsine (ED) · Methyldichloroarsine (MD) · Phenyldichloroarsine (PD) · Lewisite (L) · Sulfur mustard (HD · H · HT · HL · HQ) · Nitrogen mustard (HN1 · HN2 · HN3)
Nerve Pulmonary Incapacitating Riot control Categories:- Pulmonary agents
- Chloroformates
Wikimedia Foundation. 2010.
