- 1,2-Dichloroethane
-
1,2-Dichloroethane 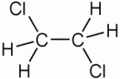
 1,2-DichloroethaneOther namesEthylene dichloride
1,2-DichloroethaneOther namesEthylene dichloride
Ethane dichloride
Dutch liquid, Dutch oil
Freon 150Identifiers CAS number 107-06-2 
ChemSpider 10 
UNII 55163IJI47 
KEGG C06752 
ChEMBL CHEMBL16370 
RTECS number KI0525000 Jmol-3D images Image 1 - ClCCCl
Properties Molecular formula C2H4Cl2 Molar mass 98.96 g mol−1 Appearance Colorless liquid with
characteristic odorDensity 1.253 g/cm³, liquid Melting point -35 °C, 238 K, -31 °F
Boiling point 84 °C, 357 K, 183 °F
Solubility in water 0.87 g/100 mL (20 °C) Viscosity 0.84 mPa·s at 20 °C Structure Dipole moment 1.80 D Hazards MSDS External MSDS R-phrases R11 R45 R36/37/38 S-phrases S45 S53 Main hazards Toxic, flammable, corrosive NFPA 704 Flash point 13 °C Related compounds Related haloalkanes Methyl chloride
Methylene chloride
1,1,1-TrichloroethaneRelated compounds Ethylene
Chlorine
Vinyl chlorideSupplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references The chemical compound 1,2-dichloroethane, commonly known by its old name of ethylene dichloride (EDC), is a chlorinated hydrocarbon, mainly used to produce vinyl chloride monomer (VCM, chloroethene), the major precursor for PVC production. It is a colourless liquid with a chloroform-like odour. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds and as a solvent. It forms azeotropes with many other solvents, including water (b.p. 70.5 C) and other chlorocarbons.[1]
Contents
History
In 1794, physician Jan Rudolph Deiman, merchant Adriaan Paets van Troostwijk, chemist Anthoni Lauwerenburg, and botanist Nicolaas Bondt, under the name of Gezelschap der Hollandsche Scheikundigen (Dutch: Society of Dutch Chemists), were the first to produce 1,2-dichloroethane from olefiant gas (oil-making gas, ethylene) and chlorine gas. Although the Gezelschap in practice did not do much in-depth scientific research, they and their publications were highly regarded. Part of that acknowledgement is that 1,2-dichloroethane has been called "Dutch oil" in old chemistry.
Production
Nearly 20 million tons of 1,2-dichloroethane are produced in the United States, Western Europe, and Japan.[2] Production is primarily achieved through the iron(III) chloride-catalysed reaction of ethene (ethylene) and chlorine.
- H2C=CH2 + Cl2 → ClCH2-CH2Cl
1,2-dichloroethane is also generated by the copper(II) chloride-catalysed "oxychlorination" of ethylene:
- 2 H2C=CH2 + 4 HCl + O2 → 2 ClCH2-CH2Cl + 2 H2O
In principle, it can be prepared by the chlorination of ethane and, less directly, from ethanol.
Uses
Vinyl chloride monomer (VCM) production
With approximately 80% of the world's consumption of 1,2-dichloroethane, the major use of 1,2-dichloroethane is in the production of vinyl chloride monomer (VCM, chloroethene) with hydrogen chloride as a byproduct. VCM is the precursor to polyvinyl chloride.
- Cl-CH2-CH2-Cl → H2C=CH-Cl + HCl
The hydrogen chloride can be re-used in the production of more 1,2-dichloroethane via the oxychlorination route described above.
Other uses
As a good apolar aprotic solvent, 1,2-dichloroethane is used as degreaser and paint remover. As a useful 'building block' reagent, it is used as an intermediate in the production of various organic compounds such as ethylenediamine. In the laboratory it is occasionally used as a source of chlorine, with elimination of ethene and chloride.
Via several steps, 1,2-dichloroethane is a precursor to 1,1,1-trichloroethane, which is used in dry cleaning. Historically, 1,2-dichloroethane was used as an anti-knock additive in leaded fuels.[3]
Safety
1,2-Dichloroethane is toxic (especially by inhalation due to its high vapour pressure), corrosive, highly flammable,[4] and carcinogenic. Its high solubility and 50-year half-life in anoxic aquifers make it a perennial pollutant and health risk that is very expensive to treat conventionally, requiring a method of bioremediation.[5] While the chemical is banned from use by U.S. manufacturers[6], a case was reported in 2009 of molded plastic consumer products (toys and holiday decorations) from China that released 1,2-dichloroethane into homes at levels high enough to produce cancer risk.[7] Substitutes are recommended and will vary according to application. Dioxolane and toluene are possible substitutes as solvents. Dichloroethane is unstable in the presence of aluminium metal and, when moist, with zinc and iron.
References
- ^ Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Rassaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, Trevor Mann “Chlorinated Hydrocarbons” in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. http://dx.doi.org/10.1002/14356007.a06_233.pub2
- ^ J.A. Field & R. Sierra-Alvarez (2004). "Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds". Rev. Environ. Sci. Biotechnol. 3 (3): 185–254. doi:10.1007/s11157-004-4733-8.
- ^ Seyferth, D. (2003). "The Rise and Fall of Tetraethyllead. 2". Organometallics 22 (25): 5154–5178. doi:10.1021/om030621b.
- ^ "1,2-Dichoroethane MSDS." Mallinckrodt Chemicals. 19 May 2008. Web. <http://hazard.com/msds/mf/baker/baker/files/d2440.htm>.
- ^ S. De Wildeman & W. Verstraete (25 Mar., 2003). "The quest for microbial reductive dechlorination of C2 to C4 chloroalkanes is warranted". Appl. Microbiol. Biotechnol. 61 (2): 94–102. doi:10.1007/s00253-002-1174-6. PMID 12655450.
- ^ Toxic Christmas: Plastic Ornaments May Pollute Your Air
- ^ Doucette, WJ and Hall, AJ and Gorder, KA (Winter, 2010). "Emissions of 1, 2-Dichloroethane from Holiday Decorations as a Source of Indoor Air Contamination". Ground Water Monitoring & Remediation 30 (1): 67–73. doi:0.1111/j.1745-6592.2009.01267.x.
External links
- Gezelschap der Hollandsche Scheikundigen
- ChemicalLand compound database
- Environmental Chemistry compound database
- Merck Chemicals database
- National Pollutant Inventory - 1,2 Dichlorethane Fact Sheet
- Locating and estimating air emissions from sources of ethylene dichloride, EPA report EPA-450/4-84-007d, March 1984
Motor fuels Fuel types Gasoline/petrol • Diesel • Lead Replacement Petrol • Racing fuelFuel additives Butyl rubber • Butylated hydroxytoluene • 1,2-Dibromoethane • 1,2-Dichloroethane • Dimethyl methylphosphonate • 2,4-Dimethyl-6-tert-butylphenol • Dinonylnaphthylsulfonic acid • 2,6-Di-tert-butylphenol • Ecalene • Ethylenediamine • Metal deactivator • Methyl tert-butyl ether • Nitromethane • Tetraethyllead • TetranitromethaneFluids Retail Categories:- Hazardous air pollutants
- IARC Group 2B carcinogens
- Organochloride insecticides
- Organochlorides
- Plastics
- Halogenated solvents
- Fuel additives
Wikimedia Foundation. 2010.

