- Barbital
-
Barbital 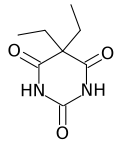
Systematic (IUPAC) name 5,5-diethylpyrimidine-2,4,6(1H,3H,5H)-trione Clinical data MedlinePlus a682221 Pregnancy cat. ? Legal status ? Routes Oral Pharmacokinetic data Half-life 30.3 (± 3.2) hours Identifiers CAS number 57-44-3 
ATC code N05CA04 PubChem CID 2294 DrugBank DB01483 ChemSpider 2206 
UNII 5WZ53ENE2P 
KEGG D01740 
ChEBI CHEBI:31252 
ChEMBL CHEMBL444 
Chemical data Formula C8H12N2O3 Mol. mass 184.193 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
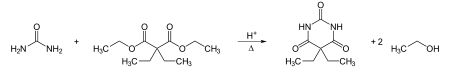
(what is this?) (verify)Barbital (marketed under the brand name Veronal), also called barbitone, was the first commercially marketed barbiturate. It was used as a sleeping aid (hypnotic) from 1903 until the mid-1950s. The chemical names for barbital are diethylmalonyl urea or diethylbarbituric acid. Veronal was prepared by condensing diethylmalonic ester with urea in the presence of sodium ethoxide, and then by adding at least two molar equivalents of ethyl iodide to the silver salt of malonylurea or possibly to a basic solution of the acid. The result was an odorless, slightly bitter, white crystalline powder.
Contents
Synthesis
Barbital was first synthesized in 1902 by German chemists Emil Fischer and Joseph von Mering. They published their discovery in 1903 and it was marketed in 1904 by the Bayer company as “Veronal”. A soluble salt of barbital was marketed by the Schering company as “Medinal.” It was dispensed for “insomnia induced by nervous excitability”.[1][unreliable source?] It was provided in either capsules or cachets. The therapeutic dose was ten to fifteen grains (0.65-0.97 grams). 3.5 to 4.4 grams is the deadly dose but sleep has also been prolonged up to ten days with recovery.
Barbital can be synthesized in a condensation reaction from urea and a diethyl malonate derivative:
Pharmacology
Veronal was considered to be a great improvement over the existing hypnotics. Its taste was slightly bitter, but an improvement over the strong, unpleasant taste of the commonly used bromides. It had few side effects. Its therapeutic dose was far below the toxic dose. However, prolonged usage resulted in tolerance to the drug, requiring higher doses to reach the desired effect. Fatal overdoses of this slow acting hypnotic were not uncommon.
Notes
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.References
- Fischer, Emil and Joseph von Mering, “Ueber eine neue Klasse von Schlafmitteln”, Therap Gegenw 44:97-101, 1903.
- "Veronal", in Finley, Ellingwood, M.D. The American Materia Medica, Therapeutics and Pharmacognosy", 1919. [2], accessed 07 Nov 2005.
Hypnotics/Sedatives (N05C) GABAA Agonists/PAMs Barbiturates: Allobarbital • Amobarbital • Aprobarbital • Barbital • Butabarbital • Butobarbital • Cyclobarbital • Ethallobarbital • Heptabarbital • Hexobarbital • Mephobarbital • Methohexital • Pentobarbital • Phenobarbital • Proxibarbal • Reposal • Secobarbital • Talbutal • Thiamylal • Thiopental • Vinbarbital • Vinylbital; Benzodiazepines: Brotizolam • Clonazepam • Cinolazepam • Climazolam • Doxefazepam • Estazolam • Flunitrazepam • Flurazepam • Flutoprazepam • Haloxazolam • Loprazolam • Lormetazepam • Midazolam • Nimetazepam • Nitrazepam • Quazepam • Temazepam • Triazolam; Carbamates: Carisoprodol • Ethinamate • Hexapropymate • Meprobamate • Methocarbamol • Procymate • Tybamate; Neuroactive Steroids: Acebrochol • Allopregnanolone • Alphadolone • Alphaxolone • Eltanolone • Ganaxolone • Hydroxydione • Minaxolone • Org 20599 • Org 21465 • Tetrahydrodeoxycorticosterone; Nonbenzodiazepines: CL-218,872 • Eszopiclone • Indiplon • JM-1232 • Lirequinil • Necopidem • Pazinaclone • ROD-188 • Saripidem • Suproclone • Suriclone • SX-3228 • U-89843A • U-90042 • Zaleplon • Zolpidem • Zopiclone; Phenols: Fospropofol • Propofol; Piperidinediones: Glutethimide • Methyprylon • Pyrithyldione • Piperidione; Quinazolinones: Afloqualone • Cloroqualone • Diproqualone • Etaqualone • Mebroqualone • Mecloqualone • Methaqualone • Methylmethaqualone • Nitromethaqualone; Others: 2-Methyl-2-butanol • Acetophenone • Acetylglycinamide chloral hydrate • Bromide (Lithium bromide, Potassium bromide, Sodium bromide) • Centalun • Chloral hydrate • Chloralose • Chloralodol • Clomethiazole • Dichloralphenazone • Ethanol (Alcohol) • Ethchlorvynol • Etomidate • Gaboxadol • Loreclezole • Methylpentynol • Metomidate • Paraldehyde • Petrichloral • Sulfonmethane • Trichloroethanol • Triclofos • Valerenic acid (Valerian)GABAB Agonists H1 Inverse agonists Antihistamines: Captodiame • Cyproheptadine • Dimenhydrinate • Diphenhydramine • Doxylamine • Hydroxyzine • Methapyrilene • Pheniramine • Promethazine • Propiomazine; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)α1-Adrenergic Antagonists Mianserin • Niaprazine • Trazodone; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)α2-Adrenergic Agonists 4-NEMD • Clonidine • Detomidine • Dexmedetomidine • Lofexidine • Medetomidine • Romifidine • Tizanidine • Xylazine5-HT2A Antagonists Eplivanserin • Niaprazine • Pruvanserin • Trazodone • Volinanserin; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)Melatonin Agonists Orexin Antagonists Others Acecarbromal • Apronal • Bromisoval • Cannabidiol (Cannabis) • Carbromal • Embutramide • Evoxine • Fenadiazole • Gabapentin • Kavalactones (Kava) • Mephenoxalone • Opiates/Opioids (Hydrocodone, Morphine (Opium), etc.) • Passion flower • Scopolamine (Mandrake) • ValnoctamideCategories:- Barbiturates
Wikimedia Foundation. 2010.