- 1-Propanol
-
1-Propanol 

Identifiers CAS number 71-23-8 
PubChem 1031 ChemSpider 1004 
UNII 96F264O9SV 
EC number 200-746-9 UN number 1274 DrugBank DB03175 KEGG C05979 
MeSH 1-Propanol ChEBI CHEBI:28831 
ChEMBL CHEMBL14687 
RTECS number UH8225000 Beilstein Reference 1098242 Gmelin Reference 25616 3DMet B00883 Jmol-3D images Image 1 - CCCO
Properties Molecular formula C3H8O Molar mass 60.1 g mol−1 Exact mass 60.057514878 g mol−1 Appearance Colorless liquid Density 803.4 mg cm−3 Melting point -126 °C, 147 K, -195 °F
Boiling point 97-98 °C, 370-371 K, 206-208 °F
log P 0.329 Vapor pressure 1.99 kPa (at 20 °C) Acidity (pKa) 16 Basicity (pKb) −2 Refractive index (nD) 1.387 Viscosity 1.938 mPa s Dipole moment 1.68 D Thermochemistry Std enthalpy of
formation ΔfHo298−302.79–−302.29 kJ mol-1 Std enthalpy of
combustion ΔcHo298−2.02156–−2.02106 MJ mol-1 Standard molar
entropy So298192.8 J K−1 mol−1 Specific heat capacity, C 143.96 J K−1 mol−1 Hazards GHS pictograms 


GHS signal word DANGER GHS hazard statements H225, H318, H336 GHS precautionary statements P210, P261, P280, P305+351+338 EU Index 603-003-00-0 EU classification  F
F  Xi
XiR-phrases R11, R41, R67 S-phrases (S2), S7, S16, S24, S26, S39 NFPA 704 Flash point 22 °C Autoignition
temperature371 °C Explosive limits 13.7% Related compounds Related compounds Butane
Propanamine
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
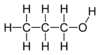
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1-Propanol is a primary alcohol with the molecular formula of C3H8O, and a structural formula of CH3CH2CH2OH. It is also known as propan-1-ol, 1-propyl alcohol, n-propyl alcohol, n-propanol, or simply propanol. It is an isomer of isopropanol (2-propanol). It is used as a solvent in the pharmaceutical industry, and for resins and cellulose esters. It is formed naturally in small amounts during many fermentation processes.
Contents
Chemical properties
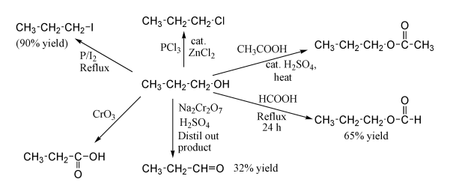
1-Propanol shows the normal reactions of a primary alcohol. Thus it can be converted to alkyl halides; for example red phosphorus and iodine produce n-propyl iodide in 80% yield, while PCl3 with catalytic ZnCl2 gives 1-chloropropane. Reaction with acetic acid in the presence of an H2SO4 catalyst under Fischer esterification conditions gives propyl acetate, while refluxing propanol overnight with formic acid alone can produce propyl formate in 65% yield. Oxidation of 1-propanol with Na2Cr2O7 and H2SO4 gives only a 36% yield of propionaldehyde, and therefore for this type of reaction higher yielding methods using PCC or the Swern oxidation are recommended. Oxidation with chromic acid yields propionic acid.
Preparation
1-Propanol is a major constituent of fusel oil, a by-product formed from certain amino acids when potatoes or grains are fermented to produce ethanol. This is no longer a significant source of propanol.
1-Propanol is manufactured by catalytic hydrogenation of propionaldehyde. The propionaldehyde is itself produced via the oxo process, by hydroformylation of ethylene using carbon monoxide and hydrogen in the presence of a catalyst such as cobalt octacarbonyl or a rhodium complex.
- H2C=CH2 + CO + H2 → CH3CH2CH=O
- CH3CH2CH=O + H2 → CH3CH2CH2OH
A traditional laboratory preparation of 1-propanol involves treating n-propyl iodide with moist Ag2O.
History
1-Propanol was discovered in 1853 by Chancel, who obtained it by fractional distillation of fusel oil.
Safety
1-Propanol is thought to be similar to ethanol in its effects on human body, but 2-4 times more potent. Oral LD50 in rats is 1870 mg/kg (compared to 7060 mg/kg for ethanol). It is metabolized into propionic acid. Effects include alcoholic intoxication and high anion gap metabolic acidosis. As of 2011, only one case of lethal 1-propanol poisoning was reported.[2]
References
- ^ "1-Propanol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=1031. Retrieved 10 October 2011.
- ^ "N-PROPANOL Health-Base Assessment and Recommendation for HEAC". http://www.dir.ca.gov/dosh/DoshReg/n-Propanol%20draft%204%20%203%202%202011%20.doc.
Further reading
- Furniss, B. S.; Hannaford, A. J.; Smith, P. W. G.; Tatchell, A. R. (1989), Vogel's Textbook of Practical Organic Chemistry (5th ed.), Harlow: Longman, ISBN 0-582-46236-3
- Lide, David R., ed (2006-06-26). CRC Handbook of Chemistry and Physics, 87th Edition (87 ed.). TF-CRC. ISBN 0849304873.
- Maryadele J. O'Neil, ed (2006-11-03). The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (14 ed.). Merck. ISBN 091191000X.
- Perkin, W. H.; Kipping, F. S (1922). Organic Chemistry. London: W. & R. Chambers. ISBN 0080223540.
External links
Alcohols (0°) Primary alcohols (1°) Ethanol · 1-Propanol · Butanol/Isobutanol · 1-Pentanol · 1-Hexanol · 1-Heptanol
Fatty alcohol: Octanol (C8) · 1-Nonanol (C9) · 1-Decanol (C10) · Undecanol (C11) · Dodecanol (C12) · 1-Tetradecanol (C14) · Cetyl alcohol (C16) · Stearyl alcohol (C18) · Arachidyl alcohol (C20) · Docosanol (C22) · Tetracosanol (C24) · Hexacosanol (C26) · Octanosol (C28) · Triacontanol (C30)
PolicosanolSecondary alcohols (2°) Isopropyl alcohol · 2-Butanol · 2-Hexanol · Cyclohexanol
Tertiary alcohols (3°) biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iAntiseptics and disinfectants (D08) Acridine derivatives Biguanides and amidines Phenol and derivatives Nitrofuran derivatives NitrofurazoneIodine products Quinoline derivatives Quaternary ammonium compounds Mercurial products Silver compounds Alcohols Other Categories:- Alcohols
- Alcohol solvents
Wikimedia Foundation. 2010.