- Cetylpyridinium chloride
-
Cetylpyridinium chloride 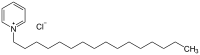 1-Hexadecylpyridinium chlorideOther namesAcetoquat CPC;
1-Hexadecylpyridinium chlorideOther namesAcetoquat CPC;
Pyrisept EXADECYL-PYRIDINIUM, CHLORIDEIdentifiers CAS number 123-03-5  ,
,
6004-24-6 (monohydrate)PubChem 31239 ChemSpider 28979 
UNII 6BR7T22E2S 
ChEBI CHEBI:32915 
ChEMBL CHEMBL34833 
ATC code B05, D08AJ03, D09AA07, R02AA06 Beilstein Reference 3578606 Jmol-3D images Image 1 - [Cl-].[n+]1(ccccc1)CCCCCCCCCCCCCCCC
- InChI=1S/C21H38N.ClH/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-22-20-17-15-18-21-22;/h15,17-18,20-21H,2-14,16,19H2,1H3;1H/q+1;/p-1

Key: YMKDRGPMQRFJGP-UHFFFAOYSA-M
InChI=1/C21H38N.ClH/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-22-20-17-15-18-21-22;/h15,17-18,20-21H,2-14,16,19H2,1H3;1H/q+1;/p-1
Key: YMKDRGPMQRFJGP-REWHXWOFAU
Properties Molecular formula C21H38ClN Molar mass 339.99 g mol−1 Appearance solid Melting point 77 °C, 350 K, 171 °F
Hazards LD50 36 mg/kg (rabbit, iv)[1]
400 mg/kg (rabbit, oral)[1]
6 mg/kg (rat, ip)[1]
30 mg/kg (rat, iv)[1]
200 mg/kg (rat, oral)[1]
250 mg/kg (rat, sc)[1]
10 mg/kg (mouse, ip)[1]
108 mg/kg (mouse, oral)[1] chloride (verify) (what is:
chloride (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cetylpyridinium chloride (CPC) is a cationic quaternary ammonium compound in some types of mouthwashes, toothpastes, lozenges, throat sprays, breath sprays, and nasal sprays. It is an antiseptic that kills bacteria and other microorganisms. It has been shown to be effective in preventing dental plaque and reducing gingivitis.[2] It has also been used as an ingredient in certain pesticides. However, this ingredient has also been alleged (according to WebMD,[3] eMedicine,[4]) to cause brown stains between the teeth and on the surface of teeth similar to chlorhexidine rinse. However, these stains can be easily removed by a dentist during a routine check-up. There have been reports of taste alteration accompanying the use of cetylpyridinium chloride mouthwash.[3] [4]
Contents
Synonyms
Cetylpyridinium chloride is present in commercial products such as 1-palmitylpyridinium chloride, C16-alkylpyridinium chloride, 1-hexadecylpyridinium chloride, acetoquat CPC, aktivex, ammonyx CPC, cecure, ceepryn chloride, cepacol, ceprim, cepacol chloride, cetafilm, cetamium, dobendan, halset, ipanol, medilave, mercocet, merothol, pionin B, pristacin, pyrisept, and asept.
Physical and chemical properties
Cetylpyridinium chloride has the molecular formula C21H38NCl and at its pure form is in a solid state at room temperature. It has a melting point of 77 °C when anhydrous or 80–83 °C in its monohydrate form. It is insoluble in acetone, acetic acid, or ethanol. It has a pyridine-like odor. It is combustible. Concentrated solutions are destructive to mucous membranes. It is toxic when swallowed and very toxic when inhaled.
In some products, cetylpyridinium bromide is used instead. Its properties are virtually identical.
Its critical micelle concentration (CMC) is 0.00124M, corresponding to 0.042% in water.
Toxicology and pharmacology
ORL-RBT LD50 400 mg kg−1[1]
IVN-RBT LD50 36 mg kg−1[1]
Compendial status
- Food Chemical Codex [5]
- United States Pharmacopeia 31 [6][clarification needed]
- British Pharmacopoeia 1998 [7][clarification needed]
See also
References
- ^ a b c d e f g h i j k Lewis, Richard J. (1996). Sax's Dangerous Properties of Industrial Materials (9th ed.). New York, NY: Van Nostrand Reinhold. p. 691.
- ^ Asadoorian, Joanna (2008). "Cetylpyridinium chloride mouth rinse on gingivitis and plaque". Journal of Dental Hygiene. http://findarticles.com/p/articles/mi_hb6368/is_5_82/ai_n31441216/.
- ^ a b "Tooth Discoloration: Causes and Treatments". http://www.webmd.com/content/article/66/79599.htm.
- ^ a b "Tooth Discoloration : Article by Jonathan A Ship, DMD". http://www.emedicine.com/derm/topic646.htm.
- ^ The United States Pharmacopeial Convention. "Revisions to FCC, First Supplement". http://www.usp.org/fcc/FCC61SBallotResultsWebPostingReport01.html. Retrieved 8 July 2009.
- ^ USP 31. "<1121> Nomenclature". http://www.usp.org/pdf/EN/USPNF/1121Nomenclature.pdf. Retrieved 8 July 2009.
- ^ Therapeutic Goods Administration. "Chemical Substances". http://www.tga.gov.au/docs/pdf/aan/aanchem.pdf. Retrieved 8 July 2009.
External links
Categories:- Quaternary ammonium compounds
- Antiseptics
- Preservatives
- Chlorides
- Pyridines
Wikimedia Foundation. 2010.
