- 1-Hexanol
-
1-Hexanol 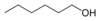

Identifiers CAS number 111-27-3 
PubChem 8103 ChemSpider 7812 
UNII 6CP2QER8GS 
EC number 203-852-3 UN number 2282 MeSH 1-Hexanol ChEMBL CHEMBL14085 
RTECS number MQ4025000 Beilstein Reference 969167 Jmol-3D images Image 1 - CCCCCCO
Properties Molecular formula C6H14O Molar mass 102.17 g mol−1 Exact mass 102.104465070 g mol−1 Density 813.6 mg cm−3 Melting point -53--41 °C, 220-232 K, -64--42 °F
Boiling point 155-159 °C, 428-432 K, 311-318 °F
Solubility in water 5.9 g dm−3 (at 20 ºC) log P 1.858 Vapor pressure 100 Pa (at 25.6 ºC) Refractive index (nD) 1.4178 (at 20 ºC) Thermochemistry Std enthalpy of
formation ΔfHo298−377.5 kJ mol−1 Std enthalpy of
combustion ΔcHo298−3.98437 MJ mol−1 Standard molar
entropy So298287.4 J K−1 mol−1 Specific heat capacity, C 243.2 J K−1 mol−1 Hazards MSDS ICSC 1084 GHS pictograms 
GHS signal word WARNING GHS hazard statements H302 EU Index 603-059-00-6 EU classification  Xn
XnR-phrases R22 S-phrases (S2), S24/25 NFPA 704 Flash point 59 °C Autoignition
temperature293 ºC  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 1-Hexanol is an organic alcohol with a six carbon chain and a condensed structural formula of CH3(CH2)5OH. This colorless liquid is slightly soluble in water, but miscible with ether and ethanol. Two additional straight chain isomers of 1-hexanol exist, 2-hexanol and 3-hexanol, both of which differ by the location of the hydroxyl group. Many isomeric alcohols have the formula C6H13OH. It is used in the perfume industry.
Contents
Preparation
Hexanol is produced industrially by the oligomerization of ethylene using triethylaluminium followed by oxidation of the alkylaluminium products.[2] An idealized synthesis is shown:
- Al(C2H5)3 + 6C2H4 → Al(C6H13)3
- Al(C6H13)3 + 1½O2 + 3H2O → 3HOC6H13 + Al(OH)3
The process generates a range of oligomers that are separated by distillation.
Alternative methods
Another method of preparation entails hydroformylation of 1-pentene followed by hydrogenation of the resulting aldehydes. This method is practiced industrially to produce mixtures of isomeric C6-alcohols, which are precursors to plasticizers.[2]
In principle, 1-hexene could be converted to 1-hexanol by hydroboration (diborane in tetrahydrofuran followed by treatment with hydrogen peroxide and sodium hydroxide):

This method is instructive and useful in laboratory synthesis but of no practical relevance because of the commercial availability of inexpensive 1-hexanol from ethylene.
Occurrence in Nature
1-hexanol is believed to be a component of the odour of freshly mown grass. Alarm pheromones emitted by the Koschevnikov gland of honey bees contain 1-hexanol.
See also
Cis-3-Hexenal, another volatile organic carbon biomolecule, also considered responsible for the freshly mowed grass flavor.
References
- ^ "1-hexanol - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification and Related Records. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8103&loc=ec_rcs. Retrieved 8 October 2011.
- ^ a b Falbe, Jürgen; Bahrmann, Helmut; Lipps, Wolfgang; Mayer, Dieter (2005), "Alcohols, Aliphatic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a01_279.
External links
Alcohols (0°) Primary alcohols (1°) Ethanol · 1-Propanol · Butanol/Isobutanol · 1-Pentanol · 1-Hexanol · 1-Heptanol
Fatty alcohol: Octanol (C8) · 1-Nonanol (C9) · 1-Decanol (C10) · Undecanol (C11) · Dodecanol (C12) · 1-Tetradecanol (C14) · Cetyl alcohol (C16) · Stearyl alcohol (C18) · Arachidyl alcohol (C20) · Docosanol (C22) · Tetracosanol (C24) · Hexacosanol (C26) · Octanosol (C28) · Triacontanol (C30)
PolicosanolSecondary alcohols (2°) Isopropyl alcohol · 2-Butanol · 2-Hexanol · Cyclohexanol
Tertiary alcohols (3°) biochemical families: prot · nucl · carb (glpr, alco, glys) · lipd (fata/i, phld, strd, gllp, eico) · amac/i · ncbs/i · ttpy/iCategories:- Flavors
- Alcohols
Wikimedia Foundation. 2010.

