- Milrinone
-
Milrinone 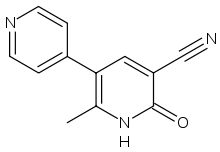
Systematic (IUPAC) name 2-methyl-6-oxo-1,6-dihydro-3,4'-bipyridine-5-carbonitrile Clinical data AHFS/Drugs.com monograph MedlinePlus a601020 Pregnancy cat. C(US) Legal status ℞ Prescription only Routes IV only Pharmacokinetic data Bioavailability 100% (as IV bolus, infusion) Protein binding 70 to 80% Metabolism Hepatic (12%) Half-life 2.3 hours (mean, in CHF) Excretion Urine (85% as unchanged drug) within 24 hours Identifiers CAS number 78415-72-2 
ATC code C01CE02 PubChem CID 4197 DrugBank APRD00010 ChemSpider 4052 
UNII JU9YAX04C7 
KEGG D00417 
ChEBI CHEBI:50693 
ChEMBL CHEMBL189 
Chemical data Formula C12H9N3O Mol. mass 211.219 g/mol SMILES eMolecules & PubChem Physical data Density 1.344 g/cm³ Melt. point 315 °C (599 °F) Boiling point 449 °C (840 °F)  (what is this?) (verify)
(what is this?) (verify)Milrinone (Primacor) is a phosphodiesterase 3 inhibitor. It potentiates the effect of cyclic adenosine monophosphate (cAMP).
Milrinone also enhances contraction of the left ventricle by increasing Ca2+-ATPase activity on the cardiac sarcoplasmic reticulum. This increases calcium ion uptake.
It has positive inotropic, vasodilating and minimal chronotropic effects. It is used in the management of heart failure only when conventional treatment with vasodilators and diuretics has proven insufficient. This is due to the potentially fatal adverse effects of milrinone, including ventricular arrhythmias.
Whereas beneficial hemodynamic effects are shown (at least short-term), several studies have shown no or a negative effect on mortality rates of hospitalized patients receiving milrinone.[1]
One negative side to the use of milrinone is the short half-life (1 to 2 hours). This can result in a prolonged weaning and possible adverse outcomes from stopping this medication rapidly.
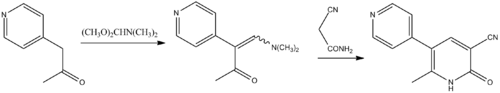
Synthesis
Singh, B.; 1983, U.S. Patent 4,413,127.
References
External links
Phosphodiesterase inhibitors PDE1 PDE2 PDE3 Amrinone • Anagrelide • Bucladesine • Cilostamide • Cilostazol • Enoximone • KMUP-1 • Milrinone • Quazinone • RPL-554 • Siguazodan • Trequinsin • Vesnarinone • ZardaverinePDE4 Arofylline • Cilomilast • CP-80633 • Denbutylline • Drotaverine • Etazolate • Filaminast • Glaucine • HT-0712 • Ibudilast • ICI-63197 • Irsogladine • Luteolin • Mesembrine • Roflumilast • Rolipram • Ro20-1724 • RPL-554 • YM-976PDE5 Acetildenafil • Aildenafil • Avanafil • Dipyridamole • Icariin • Lodenafil • Mirodenafil • MY-5445 • Sildenafil • Sulfoaildenafil • T-0156 • Tadalafil • Udenafil • VardenafilPDE6 ZaprinastPDE7 BRL-50481PDE9 BAY 73-6691 • SCH-51866PDE10 Nonselective Cardiac stimulants excluding cardiac glycosides (C01C) Adrenergic and
dopaminergic agentsαβmixedBothUnknown/ungroupedPhosphodiesterase inhibitors (PDE3I) Other cardiac stimulants 
This drug article relating to the cardiovascular system is a stub. You can help Wikipedia by expanding it.