- Luteolin
-
Luteolin 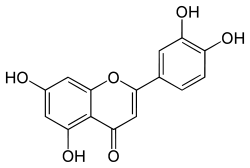 2-(3,4-Dihydroxyphenyl)- 5,7-dihydroxy-4-chromenoneOther namesLuteolol
2-(3,4-Dihydroxyphenyl)- 5,7-dihydroxy-4-chromenoneOther namesLuteolol
Digitoflavone
Flacitran
LuteolineIdentifiers CAS number 491-70-3 
PubChem 5280445 UNII KUX1ZNC9J2 
ChEMBL CHEMBL151 
Jmol-3D images Image 1 - C1=CC(=C(C=C1C2=CC (=O)C3=C(C=C(C=C3O2)O)O)O)O
Properties Molecular formula C15H10O6 Molar mass 286.24 g mol−1 Exact mass 286.047738  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Luteolin is a yellow crystalline compound. It is a flavonoid; to be specific, it is one of the more common flavones.[1] From preliminary research, it is thought to play a role in the human body possibly as an antioxidant, a free radical scavenger, a promoter of carbohydrate metabolism, or an immune system modulator[citation needed]. If applicable to the human condition, these characteristics may inhibit cancer mechanisms. Basic research results indicate luteolin as an anti-inflammatory agent[2] with other potential effects on septic shock.[citation needed] It has been suggested for multiple sclerosis on the basis of in vitro work.[3]
Luteolin is most often found in leaves, but it is also seen in celery, thyme, dandelion, rinds, barks, clover blossom, and ragweed pollen.[1] It has also been isolated from Salvia tomentosa.[4] Dietary sources include celery, green pepper, thyme, perilla, chamomile tea, carrots, olive oil, peppermint, rosemary, navel oranges, and oregano.[5][6]
Luteolin acts as a monoamine transporter activator, and is one of the few chemicals demonstrated to possess this property.[7]
Contents
Metabolism
Glycosides
- Orientin, the 8-C glucoside of luteolin
- Isoorientin, the 6-C glucoside
- Luteolin-7-glucoside (Cynaroside) and Luteolin-7-diglucoside found in dandelion coffee
- Luteolin-7-rutinoside (Veronicastroside)
Mechanism of action
Luteolin is a PDE4 inhibitor and a general phosphodiesterase inhibitor,[8] and an Interleukin 6 inhibitor.[2]
It significantly reversed the xylazine/ketamine-induced anesthesia in mice.[9]
Preclinical studies have shown that luteolin may possess pharmacological activities, including antioxidant, anti-inflammatory, antimicrobial, and anticancer activities. From preliminary research, the ability of luteolin to inhibit angiogenesis, to induce apoptosis, to affect carcinogenesis in animal models, to possibly reduce tumor growth in vivo, and to sensitize tumor cells to the cytotoxic effects of some anticancer drugs suggests that this flavonoid may have cancer chemopreventive and chemotherapeutic potential. Modulation of ROS levels, inhibition of topoisomerases I and II, reduction of NFkappaB and AP-1 activity, stabilization of p53, and inhibition of PI3K, STAT3, IGF1R, and HER2 are possible mechanisms involved in the putative biological activities of luteolin.[10]
Side-effects
Gastrointestinal adverse effects, such as nausea, vomiting and gastric hypersecretion, may occur.[citation needed]
References
- ^ a b Mann, John (1992). Secondary Metabolism (2nd ed.). Oxford, UK: Oxford University Press. pp. 279–280. ISBN 0-19-855529-6.
- ^ a b Johnson; Kelley, KW; Johnson, RW (May 2008). "Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1". Proc. Natl. Acad. Sci. U.S.A. 105 (21): 7534–9. doi:10.1073/pnas.0802865105. PMC 2396685. PMID 18490655. http://www.pnas.org/cgi/content/abstract/105/21/7534.
- ^ Theoharides (2009). "Luteolin as a Therapeutic Option for Multiple Sclerosis". Journal of Neuroinflammation 6 (1): 29. doi:10.1186/1742-2094-6-29. PMC 2768692. PMID 19825165. http://www.jneuroinflammation.com/content/6/1/29.
- ^ A. Ulubelen, M. Miski, P. Neuman, and T. J. Mabry (1979). "Flavonoids of Salvia tomentosa (Labiatae)". Journal of Natural Products 42 (4): 261–3. doi:10.1021/np50003a002.
- ^ Kayoko Shimoi, Hisae Okada, Michiyo Furugori, Toshinao Goda, Sachiko Takase, Masayuki Suzuki, Yukihiko Hara, Hiroyo Yamamoto, Naohide Kinae (1998). "Intestinal absorption of luteolin and luteolin 7-O-[beta]-glucoside in rats and humans". FEBS Letters 438 (3): 220–4. doi:10.1016/S0014-5793(98)01304-0. PMID 9827549.
- ^ López-Lázaro M. (2009). "Distribution and biological activities of the flavonoid luteolin". Mini Rev Med Chem. 9 (1): 31–59. doi:10.2174/138955709787001712. PMID 19149659.
- ^ Zhao, G; Qin, GW; Wang, J; Chu, WJ; Guo, LH (2010). "Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt". Neurochemistry international 56 (1): 168–76. doi:10.1016/j.neuint.2009.09.015. PMID 19815045.
- ^ Yu MC, Chen JH, Lai CY, Han CY, Ko WC (February 2010). "Luteolin, a non-selective competitive inhibitor of phosphodiesterases 1-5, displaced [3H-rolipram from high-affinity only rolipram-binding sites and reversed xylazine/ketamine-induced anesthesia"]. Eur. J. Pharmacol. 627 (1–3): 269–75. doi:10.1016/j.ejphar.2009.10.031. PMID 19853596. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(09)00922-4.
- ^ Yu, MC; Chen, JH; Lai, CY; Han, CY; Ko, WC (2010). "Luteolin, a non-selective competitive inhibitor of phosphodiesterases 1-5, displaced 3H-rolipram from high-affinity rolipram-binding sites and reversed xylazine/ketamine-induced anesthesia". European journal of pharmacology 627 (1–3): 269–75. doi:10.1016/j.ejphar.2009.10.031. PMID 19853596.
- ^ López-Lázaro M. (2009). "Distribution and biological activities of the flavonoid luteolin". Mini Rev Med Chem. 9 (1): 31–59. doi:10.2174/138955709787001712. PMID 19149659.
Flavones O-methylated flavones Acacetin | Diosmetin | Eupatilin | Genkwanin | Nepetin | Nobiletin | Oroxylin A | Sinensetin | Tangeritin | Techtochrysin | Tricin | WogoninGlycosides acetylated Artoindonesianin PSynthetic Categories:- Flavones
- Catechols
- Resorcinols
- PDE4 inhibitors
- Antioxidants
Wikimedia Foundation. 2010.
