- Chrysin
-
Chrysin 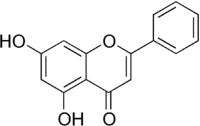 5,7-dihydroxy-2-phenyl-4H-chromen-4-oneOther names5,7-dihydroxyflavone, Chrysin, 5,7-dihydroxy-2-phenyl-(9CI), NP-005901, galangin flavanone
5,7-dihydroxy-2-phenyl-4H-chromen-4-oneOther names5,7-dihydroxyflavone, Chrysin, 5,7-dihydroxy-2-phenyl-(9CI), NP-005901, galangin flavanoneIdentifiers CAS number 480-40-0 
PubChem 5281607 ChemSpider 4444926 
UNII 3CN01F5ZJ5 
KEGG C10028 
ChEMBL CHEMBL117 
Jmol-3D images Image 1 - O=C\1c3c(O/C(=C/1)c2ccccc2)cc(O)cc3O
Properties Molecular formula C15H10O4 Molar mass 254.24 g mol−1 Exact mass 254.057909 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chrysin is a naturally occurring flavone chemically extracted from the blue passion flower (Passiflora caerulea). Honeycomb also contains small amounts. It is also reported in Oroxylum indicum or Indian trumpetflower.
Contents
Aromatase inhibition
Advertised as an aromatase inhibitor supplement by bodybuilders and athletes.[1][2] However, studies done in vivo show that orally administered chrysin does not have clinical aromatase inhibitor activity.[3][4] This has led to some practitioners administering the drug transdermally(through the skin).
Since chrysin is available as an herbal supplement, some users, for instance body builders, are taking chrysin with the hope of raising testosterone levels or stimulating testosterone production. One study listed below did not find chrysin supplementation to lead to any significant increase in testosterone production.
Chrysin was once believed to be an effective aromatase inhibitor, decreasing the levels of estrogen in the body. However, there is growing consensus that chrysin has no effect on estrogen levels in either animals or humans.[5] Early evidence was reported in the early 1980s through in vitro studies (in the laboratory, as opposed to in the body).[6][7][8][9][10][11][12] Unfortunately, follow-up studies determined that cell membranes effectively block chrysin from entering the cells and having any effect at all on estrogen levels in biological organisms.[8][13][3] In vivo (in the body) studies involving biological organisms lend support to the observation that chrysin has no effect on estrogen levels, but may have other detrimental effects to the body, particularly to thyroid function.[14] For instance, a 30 day study administered chrysin to four groups of mice both orally and via injection to examine chrysin's effect on serum estrogen levels. The results showed that chrysin had no effect on estrogen levels. Further, the mice treated with chrysin became considerably fatter, possibly due to chrysin's ability to disrupt thyroid function.[15] Another study on rats administered 50 mg of chrysin per kg body weight, considerably more than found in dietary supplements. Chrysin was found to have no ability to inhibit aromatase, possibly due to poor absorption or bioavailability.[3]
Pharmacokinetics
- Peak plasma chrysin concentrations after oral dose of 400 mg = 3–16 ng mL−1 [16]
- AUC = 5–193 ng mL−1 h [16]
- Plasma chrysin sulfate concentrations were 30-fold higher (AUC 450–4220 ng mL−1 h).[16]
- Excretion: urine peak concentration = 0.2–3.1 mg. Most of the dose appeared in faeces as chrysin.[16]
Inflammation
Chrysin has been shown to induce an anti-inflammatory effect, most likely by inhibition of COX-2 expression and via IL-6 signaling.[17]
Anxiety
In rodent in vivo studies, chrysin was found anxiolytic.[18][19]
In herbal medicine, chrysin is recommended as a remedy for anxiety,[20] but there are no controlled data in humans available.
Many herbal remedies that contain chrysin promote their value as a libido-increasing supplement. There is some in-vivo evidence for chrysin's libido-enhancing effects in rats.[21]
Chrysin demonstrated cell toxicity and inhibition of DNA synthesis at very low concentrations in a normal trout liver cell line.[22]
References
- ^ van Meeuwen JA, Korthagen N, de Jong PC, Piersma AH, van den Berg M. (2007). "(Anti)estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture". Toxicol Appl Pharmacol. 221 (3): 372–83. doi:10.1016/j.taap.2007.03.016. PMID 17482226.
- ^ Kellis JT Jr, Vickery LE. (1984). "Inhibition of human estrogen synthetase (aromatase) by flavones". Science 225 (4666): 1032–4. doi:10.1126/science.6474163. PMID 6474163.
- ^ a b c Saarinen N, Joshi SC, Ahotupa M, Li X, Ammälä J, Mäkelä S, Santti R. (2001). "No evidence for the in vivo activity of aromatase-inhibiting flavonoids". J Steroid Biochem Mol Biol. 78 (3): 231–9. doi:10.1016/S0960-0760(01)00098-X. PMID 11595503.
- ^ Int J Sport Nutr Exerc Metab. (2000). "Effects of anabolic precursors on serum testosterone concentrations and adaptations to resistance training in young men". Int J Sport Nutr Exerc Metab. 10 (3): 340–59. PMID 10997957.
- ^ Dean, W.Chrysin: Is It An Effective Aromatase Inhibitor? http://www.vrp.com/articles.aspx?page=LIST&ProdID=1208&qid=&zTYPE=2
- ^ Kellis JT, Vickery LE (September 1984). "Inhibition of human estrogen synthetase (aromatase) by flavones". Science 225 (4666): 1032–4. doi:10.1126/science.6474163. PMID 6474163. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=6474163.
- ^ Ibrahim AR, Abul-Hajj YJ (October 1990). "Aromatase inhibition by flavonoids". J. Steroid Biochem. Mol. Biol. 37 (2): 257–60. doi:10.1016/0960-0760(90)90335-I. PMID 2268557.
- ^ a b Campbell DR, Kurzer MS (September 1993). "Flavonoid inhibition of aromatase enzyme activity in human preadipocytes". J. Steroid Biochem. Mol. Biol. 46 (3): 381–8. doi:10.1016/0960-0760(93)90228-O. PMID 9831487.
- ^ Wang C, Mäkelä T, Hase T, Adlercreutz H, Kurzer MS (August 1994). "Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes". J. Steroid Biochem. Mol. Biol. 50 (3–4): 205–12. doi:10.1016/0960-0760(94)90030-2. PMID 8049151.
- ^ Pelissero C, Lenczowski MJ, Chinzi D, Davail-Cuisset B, Sumpter JP, Fostier A (February 1996). "Effects of flavonoids on aromatase activity, an in vitro study". J. Steroid Biochem. Mol. Biol. 57 (3–4): 215–23. doi:10.1016/0960-0760(95)00261-8. PMID 8645631. http://linkinghub.elsevier.com/retrieve/pii/0960-0760(95)00261-8.
- ^ Le Bail JC, Laroche T, Marre-Fournier F, Habrioux G (November 1998). "Aromatase and 17β-hydroxysteroid dehydrogenase inhibition by flavonoids". Cancer Lett. 133 (1): 101–6. doi:10.1016/S0304-3835(98)00211-0. PMID 9929167. http://linkinghub.elsevier.com/retrieve/pii/S0304-3835(98)00211-0.
- ^ Jeong HJ, Shin YG, Kim IH, Pezzuto JM (June 1999). "Inhibition of aromatase activity by flavonoids". Arch. Pharm. Res. 22 (3): 309–12. doi:10.1007/BF02976369. PMID 10403137.
- ^ King DS, Sharp RL, Vukovich MD, et al. (June 1999). "Effect of oral androstenedione on serum testosterone and adaptations to resistance training in young men: a randomized controlled trial". JAMA 281 (21): 2020–8. doi:10.1001/jama.281.21.2020. PMID 10359391. http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=10359391.> [see comments]
- ^ Koehrle J, Auf'mkolk M, Spanka M, Irmscher K, Cody V, Hesch RD (1986). "Iodothyronine deiodinase is inhibited by plant flavonoids". Prog. Clin. Biol. Res. 213: 359–71. PMID 3086894.
- ^ Shibayama, J. The Oral Bioavailability and In Vivo Activity of Chrysin in Exercising and Non-Exercising Mice. Submitted for publication, as reported by VRP article (by W. Dean)
- ^ a b c d Walle T, Otake Y, Brubaker JA, Walle UK, Halushka PV (February 2001). "Disposition and metabolism of the flavonoid chrysin in normal volunteers". Br J Clin Pharmacol 51 (2): 143–6. PMC 2014445. PMID 11259985. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2014445.
- ^ Woo KJ, Jeong YJ, Inoue H, Park JW, Kwon TK (January 2005). "Chrysin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression through the inhibition of nuclear factor for IL-6 (NF-IL6) DNA-binding activity". FEBS Lett. 579 (3): 705–11. doi:10.1016/j.febslet.2004.12.048. PMID 15670832.
- ^ Brown E, Hurd NS, McCall S, Ceremuga TE (October 2007). "Evaluation of the anxiolytic effects of chrysin, a Passiflora incarnata extract, in the laboratory rat". AANA J 75 (5): 333–7. PMID 17966676.
- ^ Wolfman C, Viola H, Paladini A, Dajas F, Medina JH (January 1994). "Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea". Pharmacol. Biochem. Behav. 47 (1): 1–4. doi:10.1016/0091-3057(94)90103-1. PMID 7906886.
- ^ Balch, Phyllis A. (2002). Prescription for herbal healing: [an easy-to-use A-to-Z reference to hundreds of common disorders and their herbal remedies]. New York: Avery. ISBN 0-89529-869-4.
- ^ Dhawan K, Kumar S, Sharma A (2002). "Beneficial effects of chrysin and benzoflavone on virility in 2-year-old male rats". J Med Food 5 (1): 43–8. doi:10.1089/109662002753723214. PMID 12511112.
- ^ Tsuji PA, Walle T. (2008). "Cytotoxic effects of the dietary flavones chrysin and apigenin in a normal trout liver cell line". Chem Biol Interact 171 (1): 37–44. doi:10.1016/j.cbi.2007.08.007. PMC 2219546. PMID 17884029. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2219546.
Flavones O-methylated flavones Acacetin | Diosmetin | Eupatilin | Genkwanin | Nepetin | Nobiletin | Oroxylin A | Sinensetin | Tangeritin | Techtochrysin | Tricin | WogoninGlycosides acetylated Artoindonesianin PSynthetic Categories:- Flavones
- Resorcinols
Wikimedia Foundation. 2010.
