- Drotaverine
-
Drotaverine 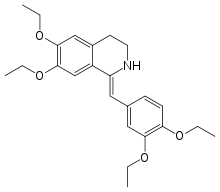
Systematic (IUPAC) name (Z)-1-(3,4-diethoxybenzylidene)-6,7-diethoxy-1,2,3,4-tetrahydroisoquinoline Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status ? Routes Oral, intravenous Pharmacokinetic data Bioavailability Highly variable Protein binding 80 to 95% Metabolism Hepatic Half-life 7 to 12 hours Excretion Fecal and renal Identifiers CAS number 985-12-6 ATC code A03AD02 PubChem CID 1712095 DrugBank DB06751 ChemSpider 1361582 
UNII 98QS4N58TW 
KEGG D07879 
ChEMBL CHEMBL551978 
Chemical data Formula C24H31NO4 Mol. mass 397.507 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Drotaverine (INN, also known as drotaverin) is an antispasmodic drug, structurally related to papaverine. Drotaverine is a selective inhibitor of phosphodiesterase 4, and has no anticholinergic effects.[citation needed] Drotaverine has been shown to possess dose-dependant analgesic effects in animal models.[1] One small study has shown drotaverine to be eliminated mainly non-renally.[2]
A few small 2003 studies found drotaverine to be nearly 80% effective in treating renal colic.[3][4] It has also been studied in accelerating labor by speeding up cervical dilation, but the results have been conflicting.[5][6][7] Drotaverine has been shown to be effective in paracervical block in managing pain during hysteroscopy and endometrial biopsy when administered together with mefenamic acid.[8] IBS patients presenting with predominant diarrhea are more likely to benefit from Buscopan.[9] Drotaverin has also been tested in combination with rimantadine for antiviral activity against A and B type influenza.[10] Drotaverin has an adverse effects frequency of 0.9%, side effects being relatively uncommon.[11]
Drotaverine in sold under brand name No-Spa (Chinoin Pharmaceutical and Chemical Works, Hungary, a member of the Sanofi-Aventis).[citation needed]
References
- ^ Stepaniuk AG, Stepaniuk NG, Stoliarchuk AA, Stepaniuk GI, Chernobrovyĭ VN (1998). "[The characteristics of the analgesic action of nitrosorbide and no-shpa]" (in Russian). Eksperimental'naia I Klinicheskaia Farmakologiia 61 (4): 17–9. PMID 9783101.
- ^ Bolaji OO, Onyeji CO, Ogundaini AO, Olugbade TA, Ogunbona FA (1996). "Pharmacokinetics and bioavailability of drotaverine in humans". European Journal of Drug Metabolism and Pharmacokinetics 21 (3): 217–21. doi:10.1007/BF03189716. PMID 8980918.
- ^ Romics I, Molnár DL, Timberg G, et al. (July 2003). "The effect of drotaverine hydrochloride in acute colicky pain caused by renal and ureteric stones". BJU International 92 (1): 92–6. doi:10.1046/j.1464-410X.2003.04262.x. PMID 12823389.
- ^ Garmish OS, Zabashnyĭ SI, Smirnova EV, Kobeliatskiĭ IuIu (February 2003). "[Preparation no-shpa forte for the treatment of renal colic]" (in Russian). Klinichna Khirurhiia (2): 47–50. PMID 12784437.
- ^ Singh KC, Jain P, Goel N, Saxena A (January 2004). "Drotaverine hydrochloride for augmentation of labor". International Journal of Gynaecology and Obstetrics 84 (1): 17–22. doi:10.1016/S0020-7292(03)00276-5. PMID 14698825.
- ^ Madhu C, Mahavarkar S, Bhave S (July 2009). "A randomised controlled study comparing Drotaverine hydrochloride and Valethamate bromide in the augmentation of labour". Archives of Gynecology and Obstetrics 282 (1): 11–5. doi:10.1007/s00404-009-1188-8. PMID 19644697.
- ^ Gupta B, Nellore V, Mittal S (March 2008). "Drotaverine hydrochloride versus hyoscine-N-butylbromide in augmentation of labor". International Journal of Gynaecology and Obstetrics 100 (3): 244–7. doi:10.1016/j.ijgo.2007.08.020. PMID 18031745.
- ^ Sharma JB, Aruna J, Kumar P, Roy KK, Malhotra N, Kumar S (June 2009). "Comparison of efficacy of oral drotaverine plus mefenamic acid with paracervical block and with intravenous sedation for pain relief during hysteroscopy and endometrial biopsy". Indian Journal of Medical Sciences 63 (6): 244–52. doi:10.4103/0019-5359.53394. PMID 19602758.
- ^ Khalif IL, Quigley EM, Makarchuk PA, Golovenko OV, Podmarenkova LF, Dzhanayev YA (March 2009). "Interactions between symptoms and motor and visceral sensory responses of irritable bowel syndrome patients to spasmolytics (antispasmodics)". Journal of Gastrointestinal and Liver Diseases 18 (1): 17–22. PMID 19337628. http://www.jgld.ro/12009/12009_2.html.
- ^ Zhilinskaya IN, Konovalova NI, Kiselev OI, Ashmarin IP (2007). "No-Spa and Remantadin are the novel complex preparations that inhibit effectively reproduction of the avian influenza viruses". Doklady Biological Sciences 414 (1): 249–50. doi:10.1134/S0012496607030234. PMID 17668635.
- ^ Tar A, Singer J (March 2002). "[Safety profile of NO-SPA]" (in Hungarian). Orvosi Hetilap 143 (11): 559–62. PMID 12583325.
Drugs for functional gastrointestinal disorders (A03) Drugs for
functional bowel disordersTertiary
amino groupQuaternary ammonium
compoundsBenzilone • Mepenzolate • Pipenzolate • Glycopyrronium • Oxyphenonium • Penthienate • Methantheline • Propantheline • Otilonium bromide • Tridihexethyl • Isopropamide • Hexocyclium • Poldine • Bevonium • Diphemanil • Tiemonium iodide • Prifinium bromide • Timepidium bromide • FenpiveriniumPapaverine • Drotaverine • MoxaverineActing on serotonin receptorsOtherFenpiprane • Diisopromine • Chlorbenzoxamine • Pinaverium • Fenoverine • Idanpramine • Proxazole • Alverine • Trepibutone • Isometheptene • Caroverine • Phloroglucinol • Silicones • TrimethyldiphenylpropylamineBelladonna and derivatives
(antimuscarinics)tertiary amines: Atropine • Hyoscyamine
quaternary ammonium compounds: Scopolamine (Butylscopolamine, Methylscopolamine) • Methylatropine • Fentonium • Cimetropium bromidePropulsives 
This drug article relating to the gastrointestinal system is a stub. You can help Wikipedia by expanding it.
