- Roflumilast
-
Roflumilast 
Systematic (IUPAC) name 3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide Clinical data AHFS/Drugs.com Consumer Drug Information MedlinePlus a611034 Licence data EMA:Link, US FDA:link Pregnancy cat. C(US) Legal status POM (UK) ℞-only (US) Routes Oral Identifiers CAS number 162401-32-3 
ATC code R03DX07 PubChem CID 158787 ChemSpider 139677 
UNII 0P6C6ZOP5U 
ChEMBL CHEMBL193240 
Chemical data Formula C17H14Cl2F2N2O3 Mol. mass 403.207 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Roflumilast (trade names Daxas, Daliresp) is a drug which acts as a selective, long-acting inhibitor of the enzyme PDE-4. It has antiinflammatory effects and is under development as an orally administered drug for the treatment of inflammatory conditions of the lungs such as asthma, and chronic obstructive pulmonary disease (COPD).[1][2][3][4] While roflumilast was found to be effective in clinical trials, it produced several dose-limiting side effects including nausea, diarrhoea and headache, and development is continuing in an attempt to minimise the incidence of side effects while retaining clinical efficacy.[5]
In June 2010, Daxas was approved in the EU for severe COPD associated with chronic bronchitis.[6] In March 2011, Daliresp gained FDA approval in the US for reducing COPD exacerbations.[7]
Contents
Chemical synthesis
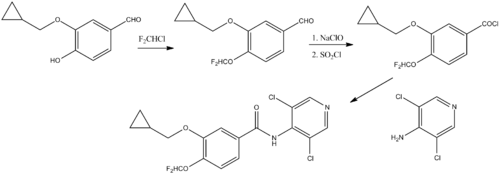
Chemical synthesis:[8]
See also
- Piclamilast
References
- ^ Boswell-Smith, V; Spina, D (2007). "PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast". International Journal of Chronic Obstructive Pulmonary Disease 2 (2): 121–9. ISSN 1178-2005. PMC 2695611. PMID 18044684. http://www.dovepress.com/getfile.php?fileID=971.
- ^ Herbert, C; Hettiaratchi, A; Webb, DC; Thomas, PS; Foster, PS; Kumar, RK (May 2008). "Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma". Clinical & Experimental Allergy 38 (5): 847–56. doi:10.1111/j.1365-2222.2008.02950.x. ISSN 1365-2222. PMID 18307529.
- ^ Hohlfeld, JM; Schoenfeld, K; Lavae-Mokhtari, M; Schaumann, F; Mueller, M; Bredenbroeker, D; Krug, N; Hermann, R (August 2008). "Roflumilast attenuates pulmonary inflammation upon segmental endotoxin challenge in healthy subjects: a randomized placebo-controlled trial". Pulmonary Pharmacology & Therapeutics 21 (4): 616–23. doi:10.1016/j.pupt.2008.02.002. ISSN 1094-5539. PMID 18374614.
- ^ Field, SK (May 2008). "Roflumilast: an oral, once-daily selective PDE-4 inhibitor for the management of COPD and asthma". Expert Opinion on Investigational Drugs 17 (5): 811–8. doi:10.1517/13543784.17.5.811. ISSN 1354-3784. PMID 18447606.
- ^ Spina, D (October 2008). "PDE4 inhibitors: current status". British Journal of Pharmacology 155 (3): 308–15. doi:10.1038/bjp.2008.307. ISSN 1476-5381. PMC 2567892. PMID 18660825. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2567892.
- ^ "Nycomed's Anti-Inflammatory Gains Approval in EU for COPD"
- ^ "FDA approves new drug to treat chronic obstructive pulmonary disease" (Press release). U.S. Food and Drug Administration (FDA). March 1, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm244989.htm.
- ^ Amschler, H.; 1998, U.S. Patent 5,712,298.
Phosphodiesterase inhibitors PDE1 PDE2 PDE3 Amrinone • Anagrelide • Bucladesine • Cilostamide • Cilostazol • Enoximone • KMUP-1 • Milrinone • Quazinone • RPL-554 • Siguazodan • Trequinsin • Vesnarinone • ZardaverinePDE4 Arofylline • Cilomilast • CP-80633 • Denbutylline • Drotaverine • Etazolate • Filaminast • Glaucine • HT-0712 • Ibudilast • ICI-63197 • Irsogladine • Luteolin • Mesembrine • Roflumilast • Rolipram • Ro20-1724 • RPL-554 • YM-976PDE5 Acetildenafil • Aildenafil • Avanafil • Dipyridamole • Icariin • Lodenafil • Mirodenafil • MY-5445 • Sildenafil • Sulfoaildenafil • T-0156 • Tadalafil • Udenafil • VardenafilPDE6 ZaprinastPDE7 BRL-50481PDE9 BAY 73-6691 • SCH-51866PDE10 Nonselective Drugs for obstructive airway diseases: asthma/COPD (R03) Adrenergics, inhalants Salbutamol#/Levosalbutamol • Fenoterol • Terbutaline • Pirbuterol • Procaterol • Bitolterol • Rimiterol • Carbuterol • Tulobuterol • ReproterolLong acting β2-agonists (LABA)otherGlucocorticoids Anticholinergics/
muscarinic antagonistMast cell stabilizers Cromoglicate • NedocromilXanthines Eicosanoid inhibition Thromboxane receptor antagonistsCombination products #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III External links
- Official website
- Daxas European Public Assessment Report (EPAR)
This drug article relating to the respiratory system is a stub. You can help Wikipedia by expanding it.