- Oleocanthal
-
Oleocanthal 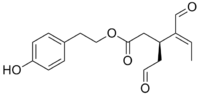 2-(4-hydroxyphenyl)ethyl (3S,4E)
2-(4-hydroxyphenyl)ethyl (3S,4E)
-4-formyl-3-(2-oxoethyl)hex-4-enoateIdentifiers CAS number 289030-99-5 ChemSpider 9827154 
Jmol-3D images Image 1 - O=CC[C@H](C(=C/C)\C=O)CC(=O)OCCc1ccc(O)cc1
- InChI=1S/C17H20O5/c1-2-14(12-19)15(7-9-18)11-17(21)22-10-8-13-3-5-16(20)6-4-13/h2-6,9,12,15,20H,7-8,10-11H2,1H3/b14-2-/t15-/m0/s1

Key: VPOVFCBNUOUZGG-VAKDEWRISA-N
InChI=1/C17H20O5/c1-2-14(12-19)15(7-9-18)11-17(21)22-10-8-13-3-5-16(20)6-4-13/h2-6,9,12,15,20H,7-8,10-11H2,1H3/b14-2-/t15-/m0/s1
Key: VPOVFCBNUOUZGG-VAKDEWRIBW
Properties Molecular formula C17H20O5 Molar mass 304.34 g/mol Density ? g/cm3  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oleocanthal is a natural organic compound isolated from extra virgin olive oil. It is responsible for the slightly peppery "bite" of extra virgin olive oil. Oleocanthal is a tyrosol ester and its chemical structure is related to oleuropein that is also found in olive oil.
Oleocanthal has been found to be have anti-inflammatory and antioxidant properties. Similar to classical NSAIDs, it is a non-selective inhibitor of cyclooxygenase (COX). It is suggested that long-term consumption of small quantities of oleocanthal from olive oil may be responsible in part for the low incidence of heart disease associated with a Mediterranean diet.[1]
50g of olive oil per day is thought to have the same effect as 1/10 of the adult ibuprofen dose.[2]
Furthermore it was shown to be an activator of the TRPA1 ion channel which is activated by many other dietary pungent compounds. Activation of TRPA1 by Oleocanthal is most likely responsible for the "peppery" taste of olive oil.[3]
See also
References
- ^ Beauchamp GK, Keast RS, Morel D, et al. (September 2005). "Phytochemistry: ibuprofen-like activity in extra-virgin olive oil". Nature 437 (7055): 45–6. doi:10.1038/437045a. PMID 16136122.
- ^ http://www.nature.com/drugdisc/news/articles/050829-11.html Extra-virgin olive oil mimics painkiller
- ^ Peyrot des Gachons C, Uchida K, Bryant B, et al. (January 2011). "Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal". J. Neurosci. 31 (3): 999–1009. doi:10.1523/JNEUROSCI.1374-10.2011. PMID 21248124.
- "Synthesis and Assignment of Absolute Configuration of (-)-Oleocanthal: A Potent, Naturally Occurring Non-steroidal Anti-inflammatory and Anti-oxidant Agent Derived from Extra Virgin Olive Oils." Smith, Amos B., III; Han, Qiang; Breslin, Paul A. S.; Beauchamp, Gary K. Organic Letters (2005), 7(22), 5075-5078.
External links
Antioxidants Acetyl-L-Carnitine (ALCAR) • Alpha-Lipoic Acid (ALA) • Ascorbic Acid (Vitamin C) • Carotenoids (Vitamin A) • Curcumin • Edaravone • Polyphenols • Glutathione • Hydroxytyrosol • L-Carnitine • Ladostigil • Melatonin • N-Acetylcysteine (NAC) • N-Acetylserotonin (NAS) • Oleocanthal • Oleuropein • Rasagiline • Resveratrol • Selegiline • Selenium • Tocopherols (Vitamin E) • Tocotrienols (Vitamin E) • Tyrosol • Ubiquinone (Coenzyme Q) • Uric AcidCategories:- Natural phenols
- Aldehydes
- Antioxidants
- Anti-inflammatory agents
- Phytochemicals
- Carboxylate esters
- Alkenes
Wikimedia Foundation. 2010.
