- 2,3-Dihydroxybenzoic acid
-
2,3-Dihydroxybenzoic acid 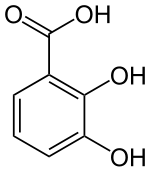 2,3-Dihydroxybenzoic acidOther names2,3-DHBA
2,3-Dihydroxybenzoic acidOther names2,3-DHBA
2,3-DHB
2-pyrocatechuic acid
o-pyrocatechuic acidIdentifiers CAS number 303-38-8 
PubChem 19 ChemSpider 18 DrugBank DB01672 KEGG C00196 
ChEMBL CHEMBL1432 
Jmol-3D images Image 1
Image 2- O=C(O)c1cccc(O)c1O
c1cc(c(c(c1)O)O)C(=O)O
Properties Molecular formula C7H6O4 Molar mass 154.12 g mol−1 Exact mass 154.026609 u Appearance Colorless solid Melting point 204-206 °C
Solubility in water low  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2,3-Dihydroxybenzoic acid is a dihydroxybenzoic acid, a type of organic compound. The colourless solid occurs naturally, being formed via the chorismic acid pathway. It is incorporated into various siderophores, which are molecules that strongly complex iron ions for absorption into bacteria. 2,3-DHB consists of a catechol group, which upon deprotonation binds iron centers very strongly, and the carboxylic acid group by which the ring attaches to various scaffolds via amide linkages. A famous high affinity siderophore is enterochelin, which contains three dihydroxybenzoyl substituents linked to the depsitripeptide of serine.[1][2] It is a potentially useful iron-chelating drug.[3]
2,3-Dihydroxybenzoic acid is also a product of human aspirin metabolism.[4]
References
- ^ I. G. O'Brien, G. B. Cox, F. Gibson (1970). "Biologically active compounds containing 2,3-dihydroxybenzoic acid and serine formed by Escherichia coli". Biochimica et Biophysica Acta 201 (3): 453–60. PMID 4908639.
- ^ Regulation of the enzymes involved in the biosynthesis of 2,3-dihydroxybenzoic acid in Aerobacter aerogenes and Escherichia coli. I.G. Young and F. Gibson, Biochimica et Biophysica Acta (BBA) - General Subjects, Volume 177, Issue 3, 6 May 1969, pages 401-411, doi:10.1016/0304-4165(69)90302-X
- ^ The identification of 2,3-dihroxybenzoic acid as a potentially useful iron-chelating drug. J. H. Graziano, R. W. Grady and A. Cerami, JPET, September 1974, vol. 190, no. 3, pages 570-575, http://jpet.aspetjournals.org/content/190/3/570.short
- ^ 2,3-Dihydroxybenzoic acid is a product of human aspirin metabolism. Martin Grootveld and Barry Halliwell, Biochemical Pharmacology, Volume 37, Issue 2, 15 January 1988, pages 271-280, doi:10.1016/0006-2952(88)90729-0
Monohydroxybenzoic acids Dihydroxybenzoic acids Gentisic acid | Homogentisic acid | Orsellinic acid | Protocatechuic acid | 2,3-Dihydroxybenzoic acid | 2,4-Dihydroxybenzoic acid | 2,6-Dihydroxybenzoic acid | 3,5-Dihydroxybenzoic acidTrihydroxybenzoic acids Bergenin | Chebulic acid | Ethyl gallate | Eudesmic acid | Gallic acid | Norbergenin | Phloroglucinol carboxylic acid | Syringic acid | TheogallinCategories:- Dihydroxybenzoic acids
- Salicylic acids
- O=C(O)c1cccc(O)c1O
Wikimedia Foundation. 2010.
