- Mepivacaine
-
Mepivacaine 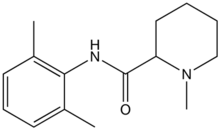
Systematic (IUPAC) name (RS)-N-(2,6-dimethylphenyl)- 1-methyl-piperidine-2-carboxamide Clinical data AHFS/Drugs.com Consumer Drug Information MedlinePlus a603026 Pregnancy cat. C, use w/ caution, may cause fetal bradycardia Legal status ? Identifiers CAS number 96-88-8 
ATC code N01BB03 PubChem CID 4062 DrugBank APRD01094 ChemSpider 3922 
UNII B6E06QE59J 
KEGG D08181 
ChEBI CHEBI:6759 
ChEMBL CHEMBL1087 
Chemical data Formula C15H22N2O Mol. mass 246.348 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mepivacaine (
 /mɛˈpɪvəkeɪn/) is a local anesthetic[1] of the amide type. Mepivacaine has a reasonably rapid onset (more rapid than that of procaine) and medium duration of action (shorter than that of procaine) and is marketed under various trade names including Carbocaine and Polocaine.
/mɛˈpɪvəkeɪn/) is a local anesthetic[1] of the amide type. Mepivacaine has a reasonably rapid onset (more rapid than that of procaine) and medium duration of action (shorter than that of procaine) and is marketed under various trade names including Carbocaine and Polocaine.Mepivacaine became available in the United States in the 1960s.
Mepivacaine is used in any infiltration and regional anesthesia.
It is supplied as the hydrochloride salt of the racemate.[2]
Chemistry
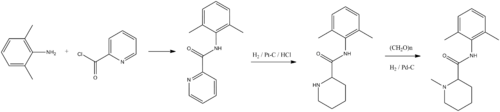
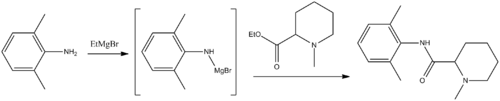
Two primary methods of synthesis have been suggested. According to the first, mepivacaine is synthesized by reacting the ethyl ester of 1-methylpiperindine-2-carboxylic acid with 2,6-dimethylanilinomagnesium bromide, which is synthesized from 2,6-dimethylaniline and ethylmagnesium bromide.
- E. Thuresson, H. Egner, U.S. Patent 2,799,679 (1957).
- B.T. Ekenstam, B. von Egner, G. Petterson, Acta Chem. Scand., 11, 1183 (1957).
- Rinderknecht, H. (1959). "Neue Lokalan�sthetika". Helvetica Chimica Acta 42 (4): 1324–1327. doi:10.1002/hlca.19590420430.
 According to the second method, reacting 2,6-dimethylaniline with the acid chloride of pyridine- carboxylic acid first gives the 2,6-xylidide of α-picolinic acid. Then the aromatic pyridine ring is reduced to piperidine by hydrogen in the presence of a platinum on carbon catalyst. The resulting 2,6-xylidide α-pipecolic acid is methylated to mepivacaine using formaldehyde with simultaneous reduction by hydrogen in the presence of platinum on carbon catalyst.
According to the second method, reacting 2,6-dimethylaniline with the acid chloride of pyridine- carboxylic acid first gives the 2,6-xylidide of α-picolinic acid. Then the aromatic pyridine ring is reduced to piperidine by hydrogen in the presence of a platinum on carbon catalyst. The resulting 2,6-xylidide α-pipecolic acid is methylated to mepivacaine using formaldehyde with simultaneous reduction by hydrogen in the presence of platinum on carbon catalyst.- B.G. Petterson, U.S. Patent 4,110,331 (1977).
References
- ^ Porto GG, Vasconcelos BC, Gomes AC, Albert D (January 2007). "Evaluation of lidocaine and mepivacaine for inferior third molar surgery". Med Oral Patol Oral Cir Bucal 12 (1): E60–4. PMID 17195831. http://www.medicinaoral.com/medoralfree01/v12i1/medoralv12i1p60.pdf.
- ^ Burm AG, Cohen IM, van Kleef JW, Vletter AA, Olieman W, Groen K (January 1997). "Pharmacokinetics of the enantiomers of mepivacaine after intravenous administration of the racemate in volunteers". Anesth. Analg. 84 (1): 85–9. doi:10.1097/00000539-199701000-00016. PMID 8989005. http://www.anesthesia-analgesia.org/cgi/pmidlookup?view=long&pmid=8989005.
External links
Anesthetics: Local anesthetics - primarily sodium channel blockers (N01B) Esters Esters of aminobenzoic acidAmylocaine • Benzocaine • Butacaine • Butamben • Chloroprocaine • Dimethocaine • Meprylcaine • Metabutoxycaine • Orthocaine • Propoxycaine • Procaine (Novocaine) • Proxymetacaine • Risocaine • TetracaineEsters of benzoic acidAmides Articaine • Bupivacaine # /Levobupivacaine/Ropivacaine • Carticaine • Cinchocaine • Etidocaine • Lidocaine # • Mepivacaine • Prilocaine • TrimecaineCombinations 
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it.