- Chloroprocaine
-
Chloroprocaine 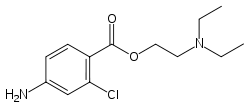
Systematic (IUPAC) name 2-diethylaminoethyl-4-amino-2-chloro-benzoate Clinical data AHFS/Drugs.com Micromedex Detailed Consumer Information Pregnancy cat. ? Legal status ? Identifiers CAS number 133-16-4 
ATC code N01BA04 PubChem CID 8612 DrugBank APRD00404 ChemSpider 8293 
UNII 5YVB0POT2H 
KEGG D07678 
ChEBI CHEBI:3636 
ChEMBL CHEMBL1179047 
Chemical data Formula C13H19ClN2O2 Mol. mass 270.755 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Chloroprocaine (trade name Nesacaine, Nesacaine-MPF) (often in the hydrochloride salt form as the aforementioned trade names) is a local anesthetic given by injection during surgical procedures and labor and delivery. Chloroprocaine constricts blood vessels resulting in reduced blood loss; this is in contrast to other local anesthetics e.g. lidocaine, which do not do such. Chloroprocaine is an ester anesthetic.[1]
Obstetrics
Amide-linked local anesthetic agents, such as lidocaine and bupivacaine, can become "trapped" in their ionized forms on the fetal side of the placenta, and therefore their net transfer across the placenta is increased. An ester-linked local anesthetic agent, 2-chloroprocaine, is rapidly metabolized, and placental transfer is limited. Since the metabolism of 2-chloroprocaine by fetal plasma is slower than in maternal plasma, the potential for ion trapping exists. Fetal pH is slightly lower than maternal (7.32 to 7.38), thus most unionized drugs are "ion trapped" to a degree, even in a healthy fetus. Chloroprocaine (pKa 8.7) is the drug of choice for epidural analgesia and a decompensating fetus, because it does not participate in ion trapping. Placental transfer of 2-chloroprocaine is not influenced by fetal acidosis.[2]
In vitro half-life of Chloroprocaine is 21 seconds for maternal and 43 seconds for fetal blood. In patients who are homozygous atypical for plasma cholinesterase, Chloroprocaine typically exists for two minutes in circulation.[3][4]
Chloroprocaine should rarely be used in the subarachnoid space because of the risk of adhesive arachnoiditis. This is likely related to the preservative sodium bisulfate. It is also not used in IV regional anesthesia due to the risk of thrombophlebitis.
Chemistry
Synthesis of this drug is accomplished by directly reacting the hydrochloride of the 4-amino-2-chlorbenzoic acid chloride with the hydrochloride of 2-diethylaminoethanol. The hydrochloride of 4-amino-2-chlorbenzoic acid chloride needed for synthesis is synthesized by reacting 2-chloro-4-aminobenzoic acid with thionyl chloride.
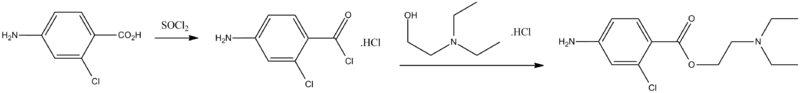
- H.C. Marks, H.I. Rubin, U.S. Patent 2,460,139 (1949).
References
- ^ Drug bank entry for Chloroprocaine
- ^ Philipson EH, Kuhnert BR, Syracuse CD. Fetal acidosis, 2-chloroprocaine, and epidural anesthesia for cesarean section. Am J Obstet Gynecol. 1985 Feb 1;151(3):322-4.
- ^ Chestnut: Obstetric Anesthesia, 3rd ed, p333.
- ^ Hughes: Anesthesia for Obstetrics, 4th ed, p75.
Anesthetics: Local anesthetics - primarily sodium channel blockers (N01B) Esters Amylocaine • Benzocaine • Butacaine • Butamben • Chloroprocaine • Dimethocaine • Meprylcaine • Metabutoxycaine • Orthocaine • Propoxycaine • Procaine (Novocaine) • Proxymetacaine • Risocaine • TetracaineAmides Articaine • Bupivacaine # /Levobupivacaine/Ropivacaine • Carticaine • Cinchocaine • Etidocaine • Lidocaine # • Mepivacaine • Prilocaine • TrimecaineCombinations 
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it.
