- Mesna
-
This article is about the sulfonate. For the thiolate MeSNa, see Sodium methanethiolate.
Mesna 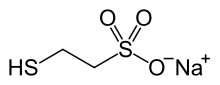
Systematic (IUPAC) name sodium 2-sulfanylethanesulfonate Clinical data AHFS/Drugs.com monograph Pregnancy cat. B1 (Au), B (U.S.) Legal status S4 (Au), POM (UK), ℞-only (U.S.) Routes Oral, intravenous Pharmacokinetic data Bioavailability 45–79% (Oral) Metabolism Oxidised in circulation Half-life 0.36–8.3 hours Excretion Renal Identifiers CAS number 19767-45-4 
ATC code R05CB05 V03AF01 PubChem CID 29769 ChemSpider 27663 
UNII NR7O1405Q9 
KEGG D01459 
ChEMBL CHEMBL975 
Chemical data Formula C2H5NaO3S2 Mol. mass 164.181 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Mesna (INN) (
 /ˈmɛznə/) is an organosulfur compound. It is used in cancer chemotherapy involving cyclophosphamide and ifosfamide as an adjuvant. It is marketed by Baxter as Uromitexan and Mesnex. MESNA is an acronym for 2-Mercaptoethane sulfonate Na (Na being the symbol for sodium).
/ˈmɛznə/) is an organosulfur compound. It is used in cancer chemotherapy involving cyclophosphamide and ifosfamide as an adjuvant. It is marketed by Baxter as Uromitexan and Mesnex. MESNA is an acronym for 2-Mercaptoethane sulfonate Na (Na being the symbol for sodium).Contents
Uses
As a chemotherapy adjuvant
Mesna is used therapeutically to reduce the incidence of haemorrhagic cystitis and haematuria when a patient receives ifosfamide or cyclophosphamide for cancer chemotherapy. These two anticancer agents, in vivo, may be converted to urotoxic metabolites such as acrolein.
Mesna assists to detoxify these metabolites by reaction of its sulfhydryl group with the vinyl group. It also increases urinary excretion of cysteine.
Other
Outside North America, mesna is also used as a mucolytic agent, working in the same way as acetylcysteine; it is sold for this indication as Mistabron[1] and Mistabronco.
Administration
It is administered intravenously, but oral dosing has been investigated.[2]
Mechanism
It is believed to act as an antioxidant.[3]
References
- ^ "Mistabron Ampoules". South African Electronic Package Inserts. August 1973. http://home.intekom.com/pharm/ucb/mistabrn.html. Retrieved 2008-08-12.
- ^ Mace JR, Keohan ML, Bernardy H, et al (December 2003). "Crossover randomized comparison of intravenous versus intravenous/oral mesna in soft tissue sarcoma treated with high-dose ifosfamide". Clin. Cancer Res. 9 (16 Pt 1): 5829–34. PMID 14676103. http://clincancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=14676103.
- ^ Mashiach E, Sela S, Weinstein T, Cohen HI, Shasha SM, Kristal B (March 2001). "Mesna: a novel renoprotective antioxidant in ischaemic acute renal failure". Nephrol. Dial. Transplant. 16 (3): 542–51. doi:10.1093/ndt/16.3.542. PMID 11239029. http://ndt.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11239029.
External links
Cough and cold preparations (R05) Expectorants Mucolytics Acetylcysteine • Ambroxol • Bromhexine • Carbocisteine • Domiodol • Dornase alfa • Eprazinone • Erdosteine • Letosteine • Mesna • Neltenexine • Sobrerol • Stepronin • TioproninCough suppressants Acetyldihydrocodeine • Benzylmorphine • Codeine • Dextromethorphan • Diacetylmorphine • Dihydrocodeine • Dimemorfan • Droxypropine • Ethylmorphine • Hydrocodone • Hydromorphone • Isoaminile • Laudanum • Levomethadone • Levopropoxyphene • Methadone • Nicocodeine • Nicodicodeine • Normethadone • Noscapine • Pholcodine • Thebacon • Tipepidine • ZipeprolOtherBenzonatate • Benproperine • Bibenzonium bromide • Butamirate • Clobutinol • Clofedanol • Cloperastine • Diphenhydramine • Dibunate • Dimethoxanate • Dropropizine • Fedrilate • Glaucine • Levodropropizine • Meprotixol • Morclofone • Nepinalone • Oxolamine • Oxeladin • Pentoxyverine • Pipazetate • Prenoxdiazine • PiperidioneDetoxifying agents for antineoplastic treatment (V03AF) Folic acid / methotrexate Salts of Folinic acid (calcium folinate/calcium levofolinate, sodium folinate/sodium levofolinate)Uric acid (TLS) Acrolein / nitrogen mustards MesnaIron / anthracyclines Alkylating agents Keratinocyte growth factor M: NEO
tsoc, mrkr
tumr, epon, para
drug (L1i/1e/V03)
This drug article relating to the respiratory system is a stub. You can help Wikipedia by expanding it.
