- Suxamethonium chloride
-
Succinylcholine 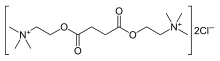
Systematic (IUPAC) name 2,2'-[(1,4-dioxobutane-1,4-diyl)bis(oxy)]bis
(N,N,N-trimethylethanaminium)Clinical data Trade names Quelicin AHFS/Drugs.com monograph Pregnancy cat. A(AU) C(US) Legal status POM (UK) ℞-only (US) Routes Intravenous, Intramuscular Pharmacokinetic data Bioavailability NA Metabolism By pseudocholinesterase, to succinylmonocholine and choline Excretion Renal (10%) Identifiers CAS number 306-40-1 
ATC code M03AB01 PubChem CID 22475 DrugBank DB00202 ChemSpider 21080 
UNII J2R869A8YF 
KEGG D00766 
ChEBI CHEBI:61219 
ChEMBL CHEMBL983 
Chemical data Formula C14H30N2O4 Mol. mass 290.399 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Suxamethonium chloride (INN), also known as suxamethonium or succinylcholine, is a paralytic drug used to induce muscle relaxation and short term paralysis, usually to facilitate tracheal intubation. Suxamethonium is sold under the trade names Anectine, Quelicin and Scoline. It is used as a paralytic agent for euthanasia/immobilization of horses. It is commonly known as "succs" in hospital (emergency department) settings.
Suxamethonium acts as a depolarizing neuromuscular blocker. It inhibits the action of acetylcholine at the neuromuscular junction, acting non-competitively on muscle type nicotinic receptors. It is degraded by butyrylcholinesterase, a plasma cholinesterase. This hydrolysis by butyrylcholinesterase is much slower than that of acetylcholine by acetylcholinesterase.
Contents
Medical uses
Its medical uses are limited to short-term muscle relaxation in anesthesia and intensive care, usually for facilitation of endotracheal intubation. Despite its adverse effects, including life threatening malignant hyperthermia, hyperkalaemia and anaphylaxis, it is perennially popular in emergency medicine because it arguably has the fastest onset and shortest duration of action of all muscle relaxants. The former is a major point of consideration in the context of trauma care, where endotracheal intubation may need to be completed very quickly. The latter means that, should attempts at endotracheal intubation fail and the patient cannot be ventilated, there is a prospect for neuromuscular recovery and the onset of spontaneous breathing before hypoxaemia occurs.
Suxamethonium is also commonly used as the sole muscle relaxant during electroconvulsive therapy, favoured for its short duration of action.
Suxamethonium is quickly degraded by plasma butyrylcholinesterase and the duration of effect is usually in the range of a few minutes. When plasma levels of butyrylcholinesterase are greatly diminished or an atypical form is present (an otherwise harmless inherited disorder), paralysis may last much longer.
Adverse effects
Side effects include malignant hyperthermia, muscle pains, acute rhabdomyolysis with hyperkalemia, transient ocular hypertension, constipation[1] and changes in cardiac rhythm including bradycardia, cardiac arrest, and ventricular dysrhythmias. In patients with neuromuscular disease or burns, a single injection of suxamethonium can lead to massive release of potassium from skeletal muscles, potentially resulting cardiac arrest. Conditions having susceptibility to suxamethonium-induced hyperkalaemia are burns, closed head injury, acidosis, Guillain–Barré syndrome, cerebral stroke, drowning, severe intraabdominal sepsis, massive trauma, myopathy, and tetanus.
Suxamethonium does not produce unconsciousness or anesthesia, and its effects may cause considerable psychological distress while simultaneously making it impossible for a patient to communicate. For these reasons, administration of the drug to a conscious patient is contraindicated, except in necessary emergency situations.
In Tamil Nadu anaesthetists ask patients for their caste because many members of the Arya Vaisya Chettiyar clan, are fatally allergic to Suxamethonium.[2]
Hyperkalemia
The side effect of hyperkalaemia happens because the acetylcholine receptor is propped open, allowing continued flow of potassium ions into the extracellular fluid. A typical increase of potassium ion serum concentration on administration of suxamethonium is 0.5 mmol per litre, whereas the normal range of potassium is 3.5 to 5 mEq per litre. The increase is transient in normal patients. Hyperkalaemia does not generally result in adverse effects below a concentration of 6.5 to 7 mEq per litre.
Severe hyperkalemia will cause changes in cardiac electrophysiology, which, if severe, can result in asystole.
Malignant hyperthermia
This side effect can result from succinylcholine administration in which a drastic and uncontrolled increase in skeletal muscleoxidative metabolism. This overwhelms the body's capacity to supply oxygen, remove carbon dioxide, and regulate body temperature, eventually leading to circulatory collapse and death if not treated quickly.
Susceptibility to MH is often inherited as an autosomal dominant disorder, for which there are at least 6 genetic loci of interest,[1] most prominently the ryanodine receptor gene (RYR1). MH susceptibility is phenotypically and genetically related to central core disease (CCD), an autosomal dominant disorder characterized both by MH symptoms and myopathy. MH is usually unmasked by anesthesia, or when a family member develops the symptoms. There is no simple, straightforward test to diagnose the condition. When MH develops during a procedure, treatment with dantrolene sodium is usually initiated; dantrolene and the avoidance of succinylcholine administration in susceptible people have markedly reduced the mortality from this condition.
Death
This drug has occasionally been used as a paralyzing agent for executions by lethal injection, although pancuronium bromide is the preferred agent today because of its longer duration of effect and its absence of fasciculations as a side effect. It has also been used for murder by Dr. Carl Coppolino.[3] Suxamethonium was the drug used to murder Nevada State Controller Kathy Augustine,[4] and was used by surgical technician Kim Hricko in the 1998 murder of her husband Steve.[5] According to Dubai authorities, Mahmoud al-Mabhouh was injected with succinylcholine by his attackers before being suffocated.[6]
Chemistry
Suxamethonium is an odourless, white crystalline substance. Aqueous solutions have a pH of about 4. The dihydrate melts at 160 °C, while the anhydrous melts at 190 °C. It is highly soluble in water (1 gram in about 1 mL), soluble in alcohol (1 gram in about 350 mL), slightly soluble in chloroform, and practically insoluble in ether. Suxamethonium is a hygroscopic compound.[7] The compound consists of two acetylcholine molecules that are linked by their acetyl groups.
History
It has been in use since the pharmacological properties of succinylcholine were discovered around 1950 by K.H. Ginzel, H Klupp, and Gerhard Werner in Vienna, Austria.
Effects
There are two phases to the blocking effect of suxamethonium; Phase 1 block is the principal paralytic effect.
Phase 1 block
Binding of suxamethonium to the nicotinic acetylcholine receptor results in opening of the receptor's monovalent cation channel; a disorganized depolarization of the motor end plate occurs and calcium is released from the sarcoplasmic reticulum.
In normal skeletal muscle, following depolarization, acetylcholine dissociates from the receptor and is rapidly hydrolyzed by acetylcholinesterase and the muscle cell is ready for the next signal.
Suxamethonium has a longer duration of effect than acetylcholine and is not hydrolyzed by acetylcholinesterase. By maintaining the membrane potential above threshold, it does not allow the muscle cell to repolarize. When acetylcholine binds to an already depolarized receptor it cannot cause further depolarization.
Calcium is removed from the muscle cell cytoplasm independent of repolarization (depolarization signaling and muscle contraction are independent processes). As the calcium is taken up by the sarcoplasmic reticulum, the muscle relaxes. This explains muscle flaccidity rather than tetany following fasciculation.
Phase 2 block
This phase isn't something abnormal and is a part of its mechanism of action. When the blood concentration of suxamethonium reaches therapeutic window, this phase starts and the muscle paralysis (Over-contractions= Loss of ATP resources => Paralysis).
References
- ^ DiPiro, Joseph, et al. Pharmacotherapy: A Pathophysiologic Approach. 6th ed. McGraw-Hill, 2005:685.
- ^ "A Chettiyar Problem". The Times of India. 30 July 2006. http://timesofindia.indiatimes.com/home/sunday-toi/A-Chettiyar-problem/articleshow/1823598.cms. Retrieved 2011-07-07.
- ^ "Trials: Tracing the Untraceable". Time. 1967-05-05. http://www.time.com/time/magazine/article/0,9171,899480,00.html. Retrieved 2010-05-24.
- ^ "Official's husband gets life for her murder". Seattle Times. June 30, 2007. http://seattletimes.nwsource.com/html/nationworld/2003768941_ndig30.html. Retrieved 2008-05-15.
- ^ "Maryland Legal Briefs, 11/23/04". The Daily Record. 2004. http://findarticles.com/p/articles/mi_qn4183/is_20041123/ai_n10064075. Retrieved 2008-09-28.
- ^ "Autopsy finds Hamas leader was drugged, suffocated, 2/28/10". CNN. 2010-02-28. http://www.cnn.com/2010/WORLD/meast/02/28/uae.murder.probe/. Retrieved 2010-02-28.
- ^ Gennaro, Alfonso. Remington: The Science and Practice of Pharmacy, 20th ed. Lippincot, Wiliams and Wilkins, 2000:1336.
Skeletal muscle relaxants (M03) Peripherally acting
(primarily antinicotinic,
NMJ block)Curare alkaloidsultra-short duration: Gantacurium
short duration: Mivacurium • Chandonium
intermediate duration: Atracurium • Cisatracurium • Fazadinium • Rocuronium • Vecuronium
long duration: Doxacurium • Dimethyltubocurarine • Pancuronium • Pipecuronium • Laudexium • Gallamine
unsorted: Hexafluronium (Hexafluorenium)Centrally acting Carbamic acid estersBenzodiazepinesAnticholinergics (Antimuscarinics)OtherBaclofen • Chlormezanone • Chlorphenesin • Chlorzoxazone • Donepezil • Eperisone • Flopropione • Mephenesin • Mephenoxalone • Metaxalone • Phenyramidol • Pridinol • Promoxolane • Quinine • Thiocolchicoside • Tizanidine • Tolperisone • TrazodoneDirectly acting Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeCategories:- Lethal injection components
- Muscle relaxants
- Nicotinic agonists
- Quaternary ammonium compounds
- Chlorides
- Succinates
- Choline esters
Wikimedia Foundation. 2010.
Look at other dictionaries:
suxamethonium chloride — sux·a·me·tho·ni·um chlo·ride (suk″sə mə thoґne əm) INN and BAN for succinylcholine chloride … Medical dictionary
Mivacurium chloride — Systematic (IUPAC) name (1R,1 R) 2,2 [[(4E) 1,8 dioxooct 4 ene 1,8 diyl]bis(oxypropane 3,1 diyl)]bis[6,7 dimethoxy 2 methyl 1 (3,4,5 trimethoxybenzyl) 1,2,3,4 tetrahydroisoquinolinium] … Wikipedia
Doxacurium chloride — Systematic (IUPAC) name bis[3 [6,7,8 trimethoxy 2 methyl 1 [(3,4,5 trimethoxyphenyl)methyl] 3,4 dihydro 1H isoquinolin 2 yl] propyl] butanedioate dichloride Clinical da … Wikipedia
Tubocurarine chloride — Systematic (IUPAC) name 6,6 dimethoxy 2,2 … Wikipedia
Dimethyltubocurarinium chloride — Systematic (IUPAC) name (1S,16R) 9,10,21,25 tetramethoxy 15,15,30,30 tetramethyl 7,23 dioxa 15,30 diazaheptacyclo[22.6.2.23,6.18,12.118,22.027,31.016,34]hexatriaconta 3,5,8(3 … Wikipedia
Ambenonium chloride — Systematic (IUPAC) name 2,2 [(1,2 dioxoethane 1,2 diyl)diimino]bis[N (2 chlorobenzyl) N,N diethylethanaminium] Clinical data AHFS … Wikipedia
Suxethonium chloride — Suxethonium (trade name: Brevidil E ) is a depolarising muscle relaxant which was presented as a dry powder in an ampoule. This was re constituted with sterile water prior to use. It was once available in Australia (and may still be but I haven t … Wikipedia
Nicotinic agonist — A nicotinic agonist is a drug which enhances the action at the nicotinic acetylcholine receptor (nAChR). Examples include: nicotine (by definition the nicotinic acetylcholine receptor is named for its affinity for nicotine) acetylcholine, the… … Wikipedia
Сукцинилхолин — (Succinylcholine) … Википедия
List of organic compounds — This page aims to list well known organic compounds, including organometallic compounds, to stimulate the creation of Wikipedia articles. Note that purely inorganic compounds, minerals, and chemical elements are not included on this list. There… … Wikipedia