- Dantrolene
-
Dantrolene 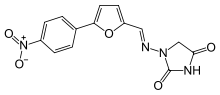
Systematic (IUPAC) name 1-{[5-(4-nitrophenyl)-2-furyl]methylideneamino}
imidazolidine-2,4-dioneClinical data Trade names Dantrium AHFS/Drugs.com monograph Pregnancy cat. C(US) Legal status ? Routes Oral, intravenous Pharmacokinetic data Bioavailability 70% Metabolism Liver Excretion Biliary, renal Identifiers CAS number 7261-97-4 
ATC code M03CA01 PubChem CID 2952 DrugBank APRD00901 ChemSpider 2847 
UNII F64QU97QCR 
KEGG D02347 
ChEBI CHEBI:4317 
ChEMBL CHEMBL1201288 
Chemical data Formula C14H10N4O5 Mol. mass 314.253 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Dantrolene sodium is a muscle relaxant that acts by abolishing excitation-contraction coupling in muscle cells, probably by action on the ryanodine receptor. It is the only specific and effective treatment for malignant hyperthermia, a rare, life-threatening disorder triggered by general anesthesia. It is also used in the management of neuroleptic malignant syndrome, muscle spasticity (e.g. after strokes, in paraplegia, cerebral palsy, or patients with multiple sclerosis), 3,4-methylenedioxymethamphetamine ("ecstasy") intoxication, serotonin syndrome, and 2,4-dinitrophenol poisoning.[1] It is marketed by JHP Pharmaceuticals LLC as Dantrium (in North America) and Dantrolen (Europe).
Contents
History
Dantrolene was first described in the scientific literature in 1967, as one of several hydantoin derivatives proposed as a new class of muscle relaxant.[2] Dantrolene underwent extensive further development, and its action on skeletal muscle was described in detail in 1973.[3]
Dantrolene was widely used in the management of spasticity before its efficacy in treating malignant hyperthermia was discovered by South African anesthesiologist Gaisford Harrison and reported in a landmark 1975 article published in the British Journal of Anaesthesia.[4] Harrison experimentally induced malignant hyperthermia with halothane anesthesia in genetically susceptible pigs, and obtained a 87.5% survival rate, where seven of his eight experiments survived after intravenous administration of dantrolene; only one animal died during the course of the study. The efficacy of dantrolene in humans was later confirmed in a large, multicenter study published in 1982.[5] Before dantrolene, the only available treatment for malignant hyperthermia was procaine, which was associated with a 60% mortality rate in animal models.[4]
Contraindications
Dantrolene cannot be used in people with:[citation needed]
- preexisting liver disease
- compromised lung function
- severe cardiovascular impairment
- known hypersensitivity to dantrolene
- pediatric patients under 5 years of age
- whenever good muscular balance/strength is needed to maintain an upright position, motoric function, or proper neuromuscular balance
If the indication is a medical emergency such as malignant hyperthermia, the only significant contraindication is hypersensitivity.[citation needed]
Pregnancy and breastfeeding
If needed in pregnancy, adequate human studies are lacking, therefore the drug should be given in pregnant women only if clearly indicated. It may cause hypotonia in the newborn if given closely before delivery.[1]
Dantrolene should not be given to breastfeeding mothers. If a treatment is necessary, breastfeeding should be terminated.[citation needed]
Adverse effects
Central nervous system side effects are quite frequently noted and encompass speech and visual disturbances, mental depression and confusion, hallucinations, headache, insomnia and exacerbation or precipitation of seizures, and increased nervousness. Infrequent cases of respiratory depression or a feeling of suffocation have been observed. Dantrolene often causes sedation severe enough to incapacitate the patient to drive or operate machinery.
Gastrointestinal effects include bad taste, anorexia, nausea, vomiting, abdominal cramps, and diarrhea.
Hepatic side effects may be seen either as asymptomatic elevation of liver enzymes and/or bilirubin or, most severe, as fatal and nonfatal hepatitis. The risk of hepatitis is associated with the duration of treatment and the daily dose. In patients treated for hyperthermia, no liver toxicity has been observed so far.
Pleural effusion with pericarditis (oral treatment only), rare cases of bone marrow damage, diffuse myalgias, backache, dermatologic reactions, transient cardiovascular reactions, and crystalluria have additionally been seen. Muscle weakness may persist for several days following treatment.
Mutagenicity and carcinogenity
Dantrolene gave positive results in animal high dose studies (with and without enzymatic activation) regarding mutagenicity and carcinogenity. No evidence for human mutagenicity and carcinogenity has been found during the long years of clinical experience.[citation needed]
Mechanism of action
Dantrolene depresses excitation-contraction coupling in skeletal muscle by binding to the ryanodine receptor, and decreasing intracellular calcium concentration.[1]
Chemistry
 Skeletal formula of azumolene. The bromine atom replacing the nitro group found in dantrolene may be seen at left.
Skeletal formula of azumolene. The bromine atom replacing the nitro group found in dantrolene may be seen at left.
Chemically it is a hydantoin derivative, but does not exhibit antiepileptic activity like other hydantoin derivates such as phenytoin.[1]
The poor water solubility of dantrolene leads to certain difficulties in its use.[1][6] A more water-soluble analog of dantrolene, azumolene, is under development for similar indications.[6] Azumolene has a bromine residue instead of the nitro group found in dantrolene, and is 30 times more water-soluble.[1]
Drug interactions
Dantrolene may interact with the following drugs:[7]
- Calcium channel blockers of the diltiazem/verapamil type: Intravenous treatment with dantrolene and concomitant calcium channel blocker treatment may lead to severe cardiovascular collapse, arrhythmias, myocardial depressions, and hyperkalemia.
- Non-depolarizing neuromuscular blocking agents, such as vecuronium bromide: Neuromuscular blockade is potentiated.
- CNS depressants: Sedative action is potentiated. Benzodiazepines may also cause additive muscle weakness.
- Combined oral contraceptives and hormone replacement therapy with estrogens: May enhance liver toxicity of dantrolene, particularly in women over 35 years of age.
References
- ^ a b c d e f Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F (2004). "Dantrolene – a review of its pharmacology, therapeutic use and new developments". Anaesthesia 59 (4): 364–73. doi:10.1111/j.1365-2044.2004.03658.x. PMID 15023108. http://www.blackwell-synergy.com/doi/full/10.1111/j.1365-2044.2004.03658.x.
- ^ Snyder HR, Davis CS, Bickerton RK, Halliday RP (September 1967). "1-[(5-arylfurfurylidene)amino]hydantoins. A new class of muscle relaxants". J Med Chem 10 (5): 807–10. doi:10.1021/jm00317a011. PMID 6048486.
- ^ Ellis KO, Castellion AW, Honkomp LJ, Wessels FL, Carpenter JE, Halliday RP (June 1973). "Dantrolene, a direct acting skeletal muscle relaxant". J Pharm Sci 62 (6): 948–51. doi:10.1002/jps.2600620619. PMID 4712630.
- ^ a b Harrison GG (January 1975). "Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium". Br J Anaesth 47 (1): 62–5. doi:10.1093/bja/47.1.62. PMID 1148076. A reprint of the article, which became a "Citation Classic", is available in Br J Anaesth 81 (4): 626–9. PMID 9924249 (free full text).
- ^ Kolb ME, Horne ML, Martz R (April 1982). "Dantrolene in human malignant hyperthermia". Anesthesiology 56 (4): 254–62. doi:10.1097/00000542-198204000-00005. PMID 7039419.
- ^ a b Sudo RT, Carmo PL, Trachez MM, Zapata-Sudo G (March 2008). "Effects of azumolene on normal and malignant hyperthermia-susceptible skeletal muscle". Basic Clin Pharmacol Toxicol 102 (3): 308–16. doi:10.1111/j.1742-7843.2007.00156.x. PMID 18047479.
- ^ "Dantrolene Drug Interactions". Epocrates Online. Epocrates. 2008. https://online.epocrates.com/u/104574/dantrolene. Retrieved on December 31, 2008.
External links
- http://www.kompendium.ch/MonographieTxt.aspx?lang=de&MonType=fi Swiss Drug Compendium on oral Dantrolene (German)
Skeletal muscle relaxants (M03) Peripherally acting
(primarily antinicotinic,
NMJ block)Curare alkaloidsultra-short duration: Gantacurium
short duration: Mivacurium • Chandonium
intermediate duration: Atracurium • Cisatracurium • Fazadinium • Rocuronium • Vecuronium
long duration: Doxacurium • Dimethyltubocurarine • Pancuronium • Pipecuronium • Laudexium • Gallamine
unsorted: Hexafluronium (Hexafluorenium)Centrally acting Carbamic acid estersBenzodiazepinesAnticholinergics (Antimuscarinics)OtherBaclofen • Chlormezanone • Chlorphenesin • Chlorzoxazone • Donepezil • Eperisone • Flopropione • Mephenesin • Mephenoxalone • Metaxalone • Phenyramidol • Pridinol • Promoxolane • Quinine • Thiocolchicoside • Tizanidine • Tolperisone • TrazodoneDirectly acting DantroleneCategories:- Muscle relaxants
- Furans
- Hydantoins
- Nitrobenzenes
Wikimedia Foundation. 2010.
