- 2,4-Dinitrophenol
-
2,4-Dinitrophenol 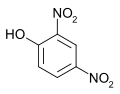
 2,4-dinitrophenolOther namesSolfo Black
2,4-dinitrophenolOther namesSolfo BlackIdentifiers CAS number 51-28-5 
PubChem 1493 ChemSpider 1448 
UNII Q13SKS21MN 
UN number 0076, 1320 DrugBank DB04528 KEGG C02496 
ChEBI CHEBI:42017 
ChEMBL CHEMBL273386 
Jmol-3D images Image 1
Image 2- O=[N+]([O-])c1cc(ccc1O)[N+]([O-])=O
c1cc(c(cc1[N+](=O)[O-])[N+](=O)[O-])O
Properties Molecular formula C6H4N2O5 Molar mass 184.106 Density 1.683 g/cm³ Melting point 108 °C, 381 K, 226 °F
Boiling point 113 °C, 386 K, 235 °F
Acidity (pKa) 4.114 Hazards R-phrases R10 R23 R24 R25 R33 S-phrases (S1) (S2) S28 S37 S45 NFPA 704  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2,4-Dinitrophenol (DNP), C6H4N2O5, is a cellular metabolic poison. It uncouples oxidative phosphorylation by carrying protons across the mitochondrial membrane, leading to a rapid consumption of energy without generation of ATP.
Dinitrophenols as a class of compounds, of which there are six members, do not occur naturally but are all manufactured compounds.
Contents
Chemical properties
2,4-Dinitrophenol is a yellow, crystalline solid that has a sweet, musty odor. It sublimes when carefully heated and is volatile with steam. It is soluble in ethyl acetate, acetone, chloroform, pyridine, carbon tetrachloride, toluene, alcohol, benzene, and aqueous alkaline solutions (Merck, 1989). Its crystalline sodium salts are also soluble in water. It forms explosive salts with alkalies and ammonia, and emits toxic fumes of nitrogen oxides when heated to decomposition (Sax, 1989). It is incompatible with heavy metals and their compounds.
Uses
Commercial DNP is primarily used for scientific research and in manufacturing. It has been used at times to make dyes, other organic chemicals, and wood preservatives. It has also been used to make photographic developer, explosives, and pesticides.
Pharmacological action
In living cells, DNP acts as a proton ionophore, an agent that can shuttle protons (hydrogen ions) across biological membranes. It defeats the proton gradient across mitochondria and chloroplast membranes, collapsing the proton motive force that the cell uses to produce most of its ATP chemical energy. Instead of producing ATP, the energy of the proton gradient is lost as heat.
DNP is often used in biochemistry research to help explore the bioenergetics of chemiosmotic and other membrane transport processes.
Dieting aid
DNP was used extensively in diet pills from 1933 to 1938 after Cutting and Tainter at Stanford University made their first report on the drug's ability to greatly increase metabolic rate.[1][2] After only its first year on the market Tainter estimated that probably at least 100,000 persons had been treated with DNP in the United States, in addition to many others abroad.[3] DNP acts as a protonophore, allowing protons to leak across the inner mitochondrial membrane and thus bypass ATP synthase. This makes ATP energy production less efficient. In effect, part of the energy that is normally produced from cellular respiration is wasted as heat. The inefficiency is proportional to the dose of DNP that's taken. As the dose increases and energy production is made more inefficient, metabolic rate increases (and more fat is burned) in order to compensate for the inefficiency and meet energy demands. DNP is probably the best known agent for uncoupling oxidative phosphorylation. The production or "phosphorylation" of ATP by ATP synthase gets disconnected or "uncoupled" from oxidation. Interestingly, the factor that limits ever-increasing doses of DNP is not a lack of ATP energy production, but rather an excessive rise in body temperature due to the heat produced during uncoupling. Accordingly, DNP overdose will cause fatal hyperthermia. In light of this, it's advised that the dose be slowly titrated according to personal tolerance, which varies greatly.[4] Case reports have shown that an acute administration of 20–50 mg/kg in humans can be lethal.[5] Concerns about dangerous side-effects and rapidly developing cataracts resulted in DNP being discontinued in the United States by the end of 1938. DNP, however, continues to be used by some bodybuilders and athletes to rapidly lose body fat. Fatal overdoses are rare, but are still reported on occasion. These include cases of accidental exposure,[6] suicide,[5][7][8] and excessive intentional exposure.[7][9][10]
There are limited and conflicting data on the pharmacokinetics of DNP in humans. The EPA states that "Data on the elimination kinetics of the dinitrophenols or their metabolic products in humans were not found."[11] The ATSDR's Toxicological Profile for Dinitrophenols also states that "No studies were located regarding distribution in humans after oral exposure to 2,4-DNP. Limited information is available regarding distribution in animals after oral exposure to 2,4-DNP." However, they do state that "Elimination from the body appears to be rapid, except possibly in cases of compromised liver function."[12] This coincides with a review in the NEJM on the biological actions of dinitrophenol, which stated that "Judging from the metabolic response, DNP appears to be eliminated entirely in three or four days; in the presence of liver or kidney damage it is possible that the drug will be retained over a longer period."[13] Oddly, more recent papers give an array of possible half-lives, ranging from 3 hours,[14] to 5–14 days.[5] Other recent papers maintain that the half-life in humans is unknown.[7]
Although further investigation is needed, one case report notes that dinitrophenol-induced hyperthermia has been successfully resolved with dantrolene administration.[15] "Dinitrophenol uncouples oxidative phosphorylation, causes release of calcium from mitochondrial stores and prevents calcium re-uptake. This leads to free intracellular calcium and causes muscle contraction and hyperthermia. Dantrolene inhibits calcium release from the sarcoplasmic reticulum which reduces intracellular calcium. The resulting muscle relaxation allows heat dissipation. There is little risk to dantrolene administration. Since dantrolene may be effective in reducing hyperthermia caused by agents that inhibit oxidative phosphorylation, early administration may improve outcome."[16]
While DNP itself is considered by many to be too risky for human use, its mechanism of action remains under investigation as a potential approach for treating obesity.[17] Currently, research is being conducted on uncoupling proteins naturally found in humans.
Environmental toxicity
DNP is considered an important environmental contaminant by the United States Environmental Protection Agency. It has been found in 61 of 1400 priority sites that need clean-up of industrial waste. It can enter the air from automobile exhaust, burning of certain industrial substances, and from reaction of nitrogen in air with other atmospheric chemicals. The major site of degradation is the soil, where microorganisms metabolize it.
However, the effects of DNP on anaerobic micro-organisms are still largely undetermined. Some studies suggest that there is anaerobic toxicity due to a reduced methane production.
References
- ^ Cutting WC, Mehrtens HG, Tainter ML (1933). "Actions and uses of dinitrophenol: Promising metabolic applications". J Am Med Assoc 101: 193–195.
- ^ Tainter ML, Stockton AB, Cutting WC (1933). "Use of dinitrophenol in obesity and related conditions: a progress report". J Am Med Assoc 101: 1472–1475.
- ^ Tainter ML, Cutting WC, Stockton AB (1934). "Use of Dinitrophenol in Nutritional Disorders : A Critical Survey of Clinical Results". Am J Public Health 24 (10): 1045–1053. doi:10.2105/AJPH.24.10.1045. PMC 1558869. PMID 18014064. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1558869.
- ^ Simkins S. (1937). "Dinitrophenol and desiccated thyroid in the treatment of obesity: a comprehensive clinical and laboratory study". J Am Med Assoc 108: 2110–2117.
- ^ a b c Hsiao AL, Santucci KA, Seo-Mayer P, et al. (2005). "Pediatric fatality following ingestion of dinitrophenol: postmortem identification of a "dietary supplement"". Clin Toxicol (Phila) 43 (4): 281–285. PMID 16035205.
- ^ Leftwich RB, Floro JF, Neal RA, Wood AJ (February 1982). "Dinitrophenol poisoning: a diagnosis to consider in undiagnosed fever". South. Med. J. 75 (2): 182–184. doi:10.1097/00007611-198202000-00016. PMID 7058360. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0038-4348&volume=75&issue=2&spage=182.
- ^ a b c A. Hahn, K. Begemann, R. Burger, J. Hillebrand, H. Meyer, K. Preußner: "Cases of Poisoning Reported by Physicians in 2006", page 40. BfR Press and Public Relations Office, 2006.
- ^ Bartlett J, Brunner M, Gough K (February 2010). "Deliberate poisoning with dinitrophenol (DNP): an unlicensed weight loss pill". Emerg Med J 27 (2): 159–160. doi:10.1136/emj.2008.069401. PMID 20156878.
- ^ McFee RB, Caraccio TR, McGuigan MA, Reynolds SA, Bellanger P (2004). "Dying to be thin: a dinitrophenol related fatality". Veterinary and human toxicology 46 (5): 251–254. PMID 15487646.
- ^ Miranda EJ, McIntyre IM, Parker DR, Gary RD, Logan BK (2006). "Two deaths attributed to the use of 2,4-dinitrophenol". Journal of analytical toxicology 30 (3): 219–222. PMID 16803658.
- ^ "Ambient water quality criteria for nitrophenols, 440/5-80-063" (PDF). U.S. Environmental Protection Agency. 1980. http://www.epa.gov/ost/pc/ambientwqc/nitrophenols80.pdf.
- ^ Harris, M. O., and Cocoran, J. J. (1995). "Toxicological Profile for Dinitrophenols". Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/toxprofiles/tp64.html.
- ^ Edsall, G. (1934). "Biological actions of dinitrophenol and related compounds: a review". New England Jour. Med. 211 (9): 385–390. doi:10.1056/NEJM193408302110901.
- ^ Korde AS, Pettigrew LC, Craddock SD, Maragos WF (September 2005). "The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia". J. Neurochem. 94 (6): 1676–1684. doi:10.1111/j.1471-4159.2005.03328.x. PMID 16045446.
- ^ Kumar S, Barker K, Seger D. Dinitrophenol-Induced Hyperthermia Resolving With Dantrolene Administration. Abstracts of the North American Congress of Clinical Toxicology. Clin Toxicol 2002; 40:599–673.
- ^ Barker K, Seger D, Kumar S (2006). "Comment on "Pediatric fatality following ingestion of Dinitrophenol: postmortem identification of a 'dietary supplement'"". Clin Toxicol (Phila) 44 (3): 351. doi:10.1080/15563650600584709. PMID 16749560.
- ^ Harper JA, Dickinson K, Brand MD (2001). "Mitochondrial uncoupling as a target for drug development for the treatment of obesity". Obesity reviews : an official journal of the International Association for the Study of Obesity 2 (4): 255–265. doi:10.1046/j.1467-789X.2001.00043.x. PMID 12119996.
Further reading
- "Food Standards Agency issues urgent advice on consumption of 'fat burner' capsules containing DNP" (Press release). Food Standards Agency. June 17, 2003). http://www.food.gov.uk/news/pressreleases/2003/jun/fatburnpress. Retrieved 2007-09-30.
External links
- "ToxFAQ about Dinitrophenols". Agency for Toxic Substances and Disease Registry. September 1996. http://www.atsdr.cdc.gov/tfacts64.html. Retrieved 2005-07-17.
- General 2,4-dinitrophenol information.
Categories:- Withdrawn drugs
- Antiobesity drugs
- Toxicology
- Phenols
- Nitrobenzenes
- Ionophores
- O=[N+]([O-])c1cc(ccc1O)[N+]([O-])=O
Wikimedia Foundation. 2010.

