- Heme
A heme (
American English ) or haem (British English ) is aprosthetic group that consists of aniron atom contained in the center of a largeheterocyclic organic ring called a "porphyrin ". Not all porphyrins contain iron, but a substantial fraction of porphyrin-containingmetalloprotein s have heme as their prosthetic subunit; these are known ashemoprotein s.Types
Major hemes
There are several biologically important kinds of heme:
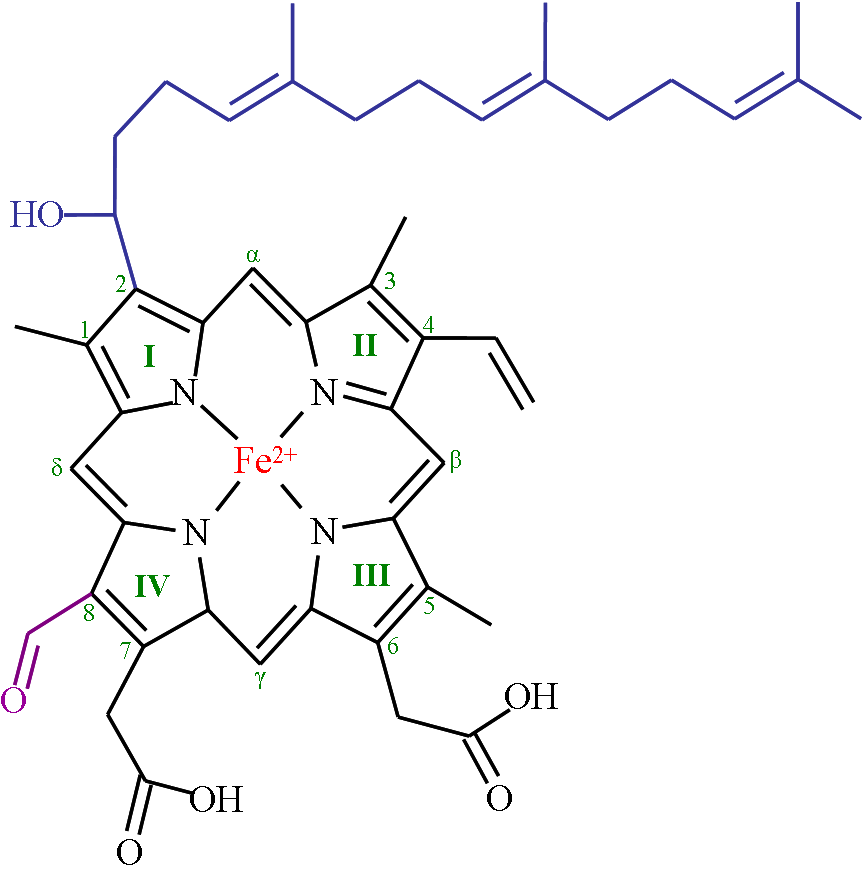
thumb|200px|right|">Heme A [ cite journal | author=Caughey, Winslow S., "et al" | title=Heme A of Cytochrome c Oxidase STRUCTURE AND PROPERTIES: COMPARISONS WITH HEMES B, C, AND S AND DERIVATIVES| journal=J. Biol. Chem.| year=1975| volume=250| issue=19| pages=7602–7622 ] Heme A is synthesized from Heme B. In two sequential reactions a 17-hydroxyethylfarnesyl moiety (blue) is added at the 2-position and an aldehyde (purple) is added at the 8-position. Nomenclature is shown in green.[ cite journal | author=Hegg, Eric L., "et al" | title=Heme A Synthase Does Not Incorporate Molecular Oxygen into the Formyl Group of Heme A| journal=Biochemistry| year=2004 | volume=43 | issue=27 | pages= 8616–8624 | doi=10.1021/bi049056m] The most common type is "
heme B "; other important types include "heme A " and "heme C ". Isolated hemes are commonly designated by capital letters while hemes bound to proteins are designated by lower case letters. Cytochrome a refers to the heme A in specific combination with membrane protein forming a portion ofcytochrome c oxidase .Other hemes
* "
Heme L " is the derivative of heme B which is covalently attached to the protein of lactoperoxidase, eosinophil peroxidase and thyroid peroxidase. The addition of peroxide with the glutamyl-375 and aspartyl-225 of lactoperoxidase forms ester bonds between these amino acid residues and the heme 1- and 5-methyl groups, respectively. Similar ester bonds with these two methyl groups are thought to form in eosinophil and thyroid peroxidases. Heme L is one important characteristic of animal peroxidases; plant peroxidases incorporate heme B. Lactoperoxidase and eosinophil peroxidase are protective enzymes responsible for the destruction of invading bacteria and virus. Thyroid peroxidase is the enzyme catalyzing the biosynthesis of the important thyroid hormones. Because lactoperoxidase destroys invading organisms in the lungs and excrement, it is thought to be an important protective enzyme.* "
Heme M " is the derivative of heme B covalently bound at the active site ofmyeloperoxidase . Heme M also contains the two ester bonds at the heme 1- and 5-methyls, much as the other mammalian peroxidases. In addition, a uniquesulfonium ion linkage between the sulfur of a methionyl aminoacid residue and the heme 2-vinyl group is formed, giving this enzyme the unique capability of easily oxidizingchloride andbromide ions. Myeloperoxidase is present in mammalian neutrophils and is responsible for the destruction of invading bacteria and virus. It also synthesizeshypobromite by "mistake" which is a known mutagenic compound.* "
Heme D " is another derivative of heme B, but in which the propionic acid side chain at the carbon of position 6, ring III is bound to this carbon both via the usual C-C bond but also by the carboxyl oxygen, giving heme D a fifth ring and a lactone. Ring III is also hydroxylated at position 5, in a conformation trans to the new lactone group. [ cite journal | author=Timkovich, R., Cork, M.S., Gennis, R.B. and Johnson, P.Y. | title=Proposed Structure of Heme d, a Prostetic Group of Bacterial Terminal Oxidases| journal=Journal of the American Chemical Society| year=1985 | volume=107 | issue=21 | pages= 6069–6075 | doi=10.1021/ja00307a041] Heme D is the site for oxygen reduction to water of many types of bacteria at low oxygen tension.* "
Heme S " is related to heme B by the having aformyl group at position 2 in place of the 2-vinyl group. Heme S is found in the hemoglobin of marine worms. The correct structures of heme B and heme S were first elucidated by German chemistHans Fischer .The names of
cytochrome s typically (but not always) reflect the kinds of hemes they contain: cytochrome a contains heme A, cytochrome c contains heme C, etc.Function
Hemoproteins have diverse biological functions including the transportation of diatomic gases, chemicalcatalysis , diatomic gas detection, and electron transfer. The heme iron serves as a source or sink of electrons during electron transfer or redox chemistry. Inperoxidase reactions, theporphyrin molecule also serves as an electron source. In the transportation or detection of diatomic gases, the gas binds to the heme iron. During the detection of diatomic gases, the binding of the gasligand to the heme iron induces conformational changes in the surrounding protein.It has been speculated that the original evolutionary function of
hemoproteins was electron transfer in primitive sulfur-basedphotosynthesis pathways in ancestralcyanobacteria before the appearance of molecular oxygen. [ cite journal | author=Hardison, R.| title=The Evolution of Hemoglobin Studies: of a very ancient protein suggest that changes in gene regulation are an important part of the evolutionary story| journal=American Scientist| year=1999 | volume=87 | issue=2 | pages= 126]Hemoproteins achieve their remarkable functional diversity by modifying the environment of the heme macrocycle within the protein matrix. For example, the ability of
hemoglobin to effectively deliver oxygen to tissues is due to specific amino acid residues located near the heme molecule. Hemoglobin binds oxygen in the pulmonary vasculature, where thepH is high and the pCO2 is low, and releases it in the tissues, where the situations are reversed. This phenomenon is known as theBohr effect . The molecular mechanism behind this effect is the steric organization of the globin chain; ahistidine residue, located adjacent to the heme group, becomes positively charged under acid circumstances, sterically releasing oxygen from the heme group.ynthesis
"Details of heme synthesis can be found in the article on
porphyrin ."The enzymatic process that produces heme is properly called
porphyrin synthesis, as all the intermediates are tetrapyrroles that are chemically classified are porphyrins. The process is highly conserved across biology. In humans, this pathway serves almost exclusively to form heme. In other species, it also produces similar substances such ascobalamin (vitamin B12 ).The pathway is initiated by the synthesis of
D-Aminolevulinic acid (dALA or δALA) from theamino acid glycine andsuccinyl-CoA from thecitric acid cycle (Krebs cycle). The rate-limiting enzyme responsible for this reaction, "ALA synthase", is strictly regulated by intracellulariron levels and heme concentration. A low-iron level, e.g., iniron deficiency , leads to decreased porphyrin synthesis, which prevents accumulation of the toxic intermediates. This mechanism is of therapeutic importance: infusion of "heme arginate" of "hematin" can abort attacks ofporphyria in patients with aninborn error of metabolism of this process, by reducing transcription of ALA synthase.The organs mainly involved in heme synthesis are the
liver and thebone marrow , although every cell requires heme to function properly. Heme is seen as an intermediate molecule in catabolism of haemoglobin in the process ofbilirubin metabolism .Degradation
In the first step, heme is converted to
biliverdin by the enzymeheme oxygenase (HOXG).NADPH is used as the reducing agent, molecular oxygen enters the reaction, carbon monoxide is produced and the iron is released from the molecule as theferric ion (Fe3+). HOXG heme --------------> biliverdin + Fe3+ / H+ + NADPH NADP+ O2 COIn the second reaction, biliverdin is converted to bilirubin by
biliverdin reductase (BVR):BVR biliverdin -----------> bilirubin / H+ + NADPH NADP+
Bilirubin is transported into the liver bound to a protein (
serum albumin ), where it is conjugated withglucuronic acid to become more water soluble. The reaction is catalyzed by the enzyme UDP-glucuronide transferase (UDPGUTF). UDPGUTF bilirubin + 2 UDP-glucuronate ------------> bilirubin diglucuronide 2 UMP + 2 PiThis form of bilirubin is excreted from the liver in
bile . Theintestinal bacteria deconjugatebilirubin diglucuronide and convert bilirubin tourobilinogen s. Some urobilinogen is absorbed by intestinal cells and transported into the kidneys and excreted with urine. The remainder travels down the digestive tract and is excreted asstercobilinogen , which is responsible for the color offeces .Genes
The following genes are part of the chemical pathway for making heme:
* "
ALAD ": aminolevulinic acid, delta-, dehydratase
* "ALAS1 ": aminolevulinate, delta-, synthase 1
* "ALAS2 ": aminolevulinate, delta-, synthase 2 (sideroblastic/hypochromic anemia)
* "CPOX ": coproporphyrinogen oxidase
* "FECH ": ferrochelatase (protoporphyria)
* "HMBS": hydroxymethylbilane synthase
* "PPOX ": protoporphyrinogen oxidase
* "UROD ": uroporphyrinogen decarboxylase
* "UROS ": uroporphyrinogen III synthase (congenital erythropoietic porphyria)ee also
*
bilirubin metabolism
*chlorin
*corrin
*cobalamin
*respiration (physiology) References
Wikimedia Foundation. 2010.