- Congo red
-
Congo red 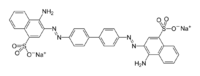 sodium sodium 3,3'-([1,1'-biphenyl]-4,4'-diyl)bis(4-aminonaphthalene-1-sulfonate)
sodium sodium 3,3'-([1,1'-biphenyl]-4,4'-diyl)bis(4-aminonaphthalene-1-sulfonate)Identifiers CAS number 573-58-0 
PubChem 11313 ChemSpider 10838 
UNII 3U05FHG59S 
MeSH Congo+red ChEMBL CHEMBL429694 
Jmol-3D images Image 1 - [Na+].[Na+].[O-]S(=O)(=O)c5cc(/N=N/c1ccc(cc1)c4ccc(/N=N/c3cc(c2ccccc2c3N)S([O-])(=O)=O)cc4)c(N)c6c5cccc6
- InChI=1S/C32H24N6O6S2.2Na/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34;;/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44);;/q;2*+1/p-2/b37-35+,38-36+;;

Key: IQFVPQOLBLOTPF-HKXUKFGYSA-L
InChI=1/C32H24N6O6S2.2Na/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34;;/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44);;/q;2*+1/p-2/b37-35+,38-36+;;
Key: IQFVPQOLBLOTPF-COCKEIKOBB
Properties Molecular formula C32H22N6Na2O6S2 Molar mass 696.665  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Congo red is the sodium salt of 3,3'-([1,1'-biphenyl]-4,4'-diyl)bis(4-aminonaphthalene-1-sulfonic acid)(formula: C32H22N6Na2O6S2; molecular weight: 696.66 g/mol). It is a secondary diazo dye. Congo red is water soluble, yielding a red colloidal solution; its solubility is better in organic solvents such as ethanol.
It has a strong, though apparently non-covalent affinity to cellulose fibers. However, the use of congo red in the cellulose industries (cotton textile, wood pulp & paper) has long been abandoned, primarily because of its tendency to change color when touched by sweaty fingers, to run, and because of its toxicity.
Contents
History
Congo red was first synthesized in 1883 by Paul Bottiger who was working then for the Friedrich Bayer Company in Elberfeld, Germany. He was looking for textile dyes that did not require a mordant step. The company was not interested in this bright red color, so he filed the patent under his name and sold it to the AGFA company of Berlin. AGFA marketed the dye under the name "Congo red", a catchy name in Germany at the time of the 1884 Berlin West Africa Conference, an important event in the Colonisation of Africa. The dye was a major commercial success for AGFA. In the following years, for the same reasons, other dyes were marketed using the "Congo" name: Congo rubine, Congo corinth, brilliant Congo, Congo orange, Congo brown, and Congo blue.[1]
Behaviour in solution
Congo red (pH indicator) below pH 3.0 above pH 5.2 3.0 ↔ 5.2 Due to a color change from blue to red at pH 3.0-5.2, congo red can be used as a pH indicator. Since this color change is an approximate inverse of that of litmus, it can be used with litmus paper in a simple parlor trick: add a drop or two of congo red to both an acid solution and a base solution. Dipping red litmus paper in the red solution will turn it blue, while dipping blue litmus paper in the blue solution will turn it red.
Congo red has a propensity to aggregate in aqueous and organic solutions. The proposed mechanisms suggest hydrophobic interactions between the aromatic rings of the dye molecules, leading to a pi-pi stacking phenomenon. Although these aggregates are present under various sizes and shapes, the "ribbon-like micelles" of a few molecules seem to be the predominant form (even if the "micelle" term is not totally appropriate here). This aggregation phenomenon is more prevalent in high congo red concentrations, at high salinity and/or low pH.
Dyeing activity
As suggested by its intense red color, congo red has important spectrophotometric properties. Indeed, its UV-visible absorption spectrum shows a characteristic, intense peak around 498 nm in aqueous solution, at low dye concentration. Congo red molar extinction coefficient is about 45000 [L]/[mol].[cm] in these conditions. Aggregation of the dye and binding to amyloid fibrils tends to red-shift the absorption spectrum, whereas binding to cellulose fibers has the opposite effect. Congo red also shows a fluorescent activity when bound to amyloid fibrils, which tends to be used as a sensitive diagnosis tool for amyloidosis, instead of the traditional histological birefringence test.
Diagnostic use
 Micrograph demonstrating amyloid deposition (red-orange) with congo red staining in cardiac amyloidosis.
Micrograph demonstrating amyloid deposition (red-orange) with congo red staining in cardiac amyloidosis.
In biochemistry and histology, congo red is used to stain microscopic preparates, especially as a cytoplasm and erythrocyte stain. Apple-green birefringence of Congo red stained preparates under polarized light is indicative for the presence of amyloid fibrils. Additionally, congo red is used in microbiological epidemiology to rapidly identify the presence of virulent serotype 2a Shigella flexneri, where the dye binds the bacterium's unique lipopolysaccharide (LPS) structure.
References
- ^ Steensma DP. 2001. "Congo" red: out of Africa? Archives of Pathology and Laboratory Medicine 125(2):250–252.
Stains Iron/Hemosiderin Lipids Carbohydrates Amyloid Congo redBacteria Gram staining (Methyl violet/Gentian violet, Safranin) · Ziehl–Neelsen stain/acid-fast (Carbol fuchsin/Fuchsine, Methylene blue) · Auramine-rhodamine stain (Auramine O, Rhodamine B)Connective tissue Other H&E stain (Haematoxylin, Eosin Y) · Silver stain (Grocott's methenamine silver stain, Warthin–Starry stain) · Methyl blue · Wright's stain · Giemsa stain · Gömöri trichrome stain · Neutral red · Janus Green BTissue stainability Categories:- PH indicators
- Azo dyes
- Staining dyes
- Naphthalenesulfonates
- 1-Naphthylamines
- Biphenyls
- Sodium compounds
Wikimedia Foundation. 2010.
