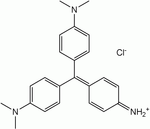
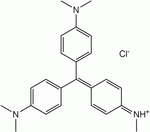
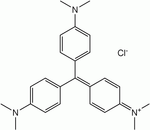
- Methyl violet
-
Methyl violet 2B 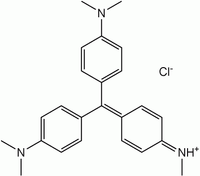 Methyl violet 2BOther namesGentian Violet B
Methyl violet 2BOther namesGentian Violet BIdentifiers CAS number 8004-87-3 
ChemSpider 170606 
Properties Molecular formula C24H28N3Cl Appearance Green to dark-green powder[1] Melting point 137 °C (279 °F) – decomposes[1]
Solubility in water Soluble in water, ethanol, insoluble in xylene[1]  violet (verify) (what is:
violet (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl violet is a family of organic compounds that are mainly used as dyes. Depending on the amount of attached methyl groups, the color of the dye can be altered. Its main use is as a purple dye for textiles and to give deep violet colors in paint and ink. Methyl violet 10B is also known as crystal violet (and many other names) and has medical uses.[2]
Contents
Structure
The term methyl violet encompasses three compounds that differ in the number of methyl groups attached to the amine functional group. They are all soluble in water, ethanol, diethylene glycol and dipropylene glycol.
Name Methyl violet 2B Methyl violet 10B (Crystal violet) Structure Formula C23H26N3Cl C24H28N3Cl C25H30N3Cl CAS no 8004-87-3 548-62-9 ChemSpider ID 21164086 170606 10588 PubChem ID 196986 11057 Formula C23H26N3 C24H28N3 C25H30N3 ChemSpider ID 2006225 3349, 9080056, 10354393 PubChem ID 2724053 3468 Methyl violet 2B
Methyl violet 2B is a green powder which is soluble in water in ethanol and water, but not in xylene. It appears yellow in solution of low pH (~0.15) and changes to violet with pH increasing toward 3.2.[1]
Methyl violet 10B
Main article: Crystal violetMethyl violet 10B has six methyl groups. It is known in medicine as Gentian violet (or crystal violet or pyoctanin(e)[2]) and is the active ingredient in a Gram stain, used to classify bacteria. It is used as a pH indicator, with a range between 0 and 1.6. The protonated form (found in acidic conditions) is yellow, turning blue-violet above pH levels of 1.6.[3] Gentian violet destroys cells and can be used as a disinfectant.[4] Compounds related to methyl violet are potential carcinogens.
Methyl violet 10B also inhibits the growth of many Gram positive bacteria, except streptococci. When used in conjunction with nalidixic acid (which destroys gram-negative bacteria), it can be used to isolate the streptococci bacteria for the diagnosis of an infection.
Methyl violet 10B also binds to DNA. This means it can be used in cell viability assays in biochemistry. However, this binding to DNA will cause replication errors in living tissue, possibly leading to mutations and cancer.
Degradation
Methyl violet is a mutagen and mitotic poison, therefore concerns exist regarding the ecological impact of the release of methyl violet into the environment. Methyl violet has been used in vast quantities for textile and paper dyeing, and 15% of such dyes produced worldwide are released to environment in wastewater. Numerous methods have been developed to treat methyl violet pollution. The three most prominent are chemical bleaching, biodegradation, and photodegradation.
Chemical bleaching
Chemical bleaching is achieved by oxidation or reduction. Oxidation can destroy the dye completely, e.g. through the use of sodium hypochlorite (NaClO, common bleach) or hydrogen peroxide.[5][6] Reduction of methyl violet occurs in microorganisms but can be attained chemically using sodium dithionite.
Biodegradation
Biodegradation has been well investigated because of its relevance to sewage plants with specialized microorganisms. Two microorganisms that have been studied in depth are the White Rot Fungus and the bacterium Nocardia Corallina.[7][8]
Photodegradation
Light alone does not rapidly degrade methyl violet,[9] but the process is accelerated upon the addition of large band-gap semiconductors, TiO2 or ZnO.[10][11]
Other methods
Many others methods have been developed to treat the contamination of dyes in a solution, including electrochemical degradation,[12] ion exchange,[13] laser degradation, and absorption onto various solids such as activated charcoal.
See also
- Fuchsine
- Methylene blue
- Methyl blue
- Egyptian Blue
- Han Purple
- Fluorescein
References
- ^ a b c d R. W. Sabnis (29 March 2010). Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications. John Wiley and Sons. pp. 309–. ISBN 9780470407530. http://books.google.com/books?id=M59Kw54ehwAC&pg=PA309. Retrieved 27 June 2011.
- ^ a b Gorgas, Ferdinand J. S. (1901). Pyoctanin – Methyl-Violet – Pyoctanine. chestofbooks.com. Archived from the original on 2011-03-15. http://chestofbooks.com/health/materia-medica-drugs/Manual-Of-Dental-Materia-Medica-And-Therapeutics/Pyoctanin-Methyl-Violet-Pyoctanine.html. Retrieved 2011-03-15.
- ^ Kristallviolett – ein pH-Indikator
- ^ WHO Model Lists of Essential Medicines, March 2007
- ^ Pizzolato, T (2002). "Colour removal with NaClO of dye wastewater from an agate-processing plant in Rio Grande do Sul, Brazil". International Journal of Mineral Processing 65: 203. doi:10.1016/S0301-7516(01)00082-5.
- ^ XP-Chloro Degradation Malachite green U.S. Patent 2,755,202
- ^ Bumpus, JA; Brock, BJ (1988). "Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium". Applied and environmental microbiology 54 (5): 1143–50. PMC 202618. PMID 3389809. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=202618.
- ^ Yatome, Chizuko; Yamada, Shigeyuki; Ogawa, Toshihiko; Matsui, Masaki (1993). "Degradation of Crystal violet by Nocardia corallina". Applied Microbiology and Biotechnology 38. doi:10.1007/BF00242956.
- ^ Bhasikuttan, A (1995). "Oxidation of crystal violet and malachite green in aqueous solutions — a kinetic spectrophotometric study". Journal of Photochemistry and Photobiology A: Chemistry 90: 177. doi:10.1016/1010-6030(95)04094-V.
- ^ Senthilkumaar, S; Porkodi, K (2005). "Heterogeneous photocatalytic decomposition of Crystal Violet in UV-illuminated sol-gel derived nanocrystalline TiO2 suspensions". Journal of colloid and interface science 288 (1): 184–9. doi:10.1016/j.jcis.2005.02.066. PMID 15927578.
- ^ Sahoo, C; Gupta, A; Pal, A (2005). "Photocatalytic degradation of Crystal Violet (C.I. Basic Violet 3) on silver ion doped TiO". Dyes and Pigments 66: 189. doi:10.1016/j.dyepig.2004.09.003.
- ^ Sanroman, M; Pazos, M; Ricart, M; Cameselle, C (2004). "Electrochemical decolourisation of structurally different dyes". Chemosphere 57 (3): 233. doi:10.1016/j.chemosphere.2004.06.019. PMID 15312740.
- ^ Wu, J; Liu, C; Chu, K; Suen, S (2008). "Removal of cationic dye methyl violet 2B from water by cation exchange membranes". Journal of Membrane Science 309: 239. doi:10.1016/j.memsci.2007.10.035.
Stains Iron/Hemosiderin Lipids Carbohydrates Amyloid Bacteria Gram staining (Methyl violet/Gentian violet, Safranin) · Ziehl–Neelsen stain/acid-fast (Carbol fuchsin/Fuchsine, Methylene blue) · Auramine-rhodamine stain (Auramine O, Rhodamine B)Connective tissue Other H&E stain (Haematoxylin, Eosin Y) · Silver stain (Grocott's methenamine silver stain, Warthin–Starry stain) · Methyl blue · Wright's stain · Giemsa stain · Gömöri trichrome stain · Neutral red · Janus Green BTissue stainability Categories:- Triarylmethane dyes
- Disinfectants
- PH indicators
- Staining dyes
- Anilines
- Chlorides
Wikimedia Foundation. 2010.