- Paraquat
-
This article is about the herbicide. For the British military operation to recapture South Georgia, see Operation Paraquat.
Paraquat 
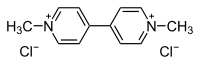 1,1'-Dimethyl-4,4'-bipyridinium dichlorideOther namesParaquat dichloride; Methyl viologen dichloride; Crisquat; Dexuron; Esgram; Gramuron; Ortho Paraquat CL; Para-col; Pillarxone; Tota-col; Toxer Total; PP148; Cyclone; Gramixel; Gramoxone; Pathclear; AH 501.
1,1'-Dimethyl-4,4'-bipyridinium dichlorideOther namesParaquat dichloride; Methyl viologen dichloride; Crisquat; Dexuron; Esgram; Gramuron; Ortho Paraquat CL; Para-col; Pillarxone; Tota-col; Toxer Total; PP148; Cyclone; Gramixel; Gramoxone; Pathclear; AH 501.Identifiers CAS number 1910-42-5 
PubChem 15938 ChemSpider 15146 
UNII 2KZ83GSS73 
ChEBI CHEBI:28786 
ChEMBL CHEMBL458019 
Jmol-3D images Image 1 - C[n+]1ccc(cc1)c2cc[n+](cc2)C.[Cl-].[Cl-]
- InChI=1S/C12H14N2.2ClH/c1-13-7-3-11(4-8-13)12-5-9-14(2)10-6-12;;/h3-10H,1-2H3;2*1H/q+2;;/p-2

Key: FIKAKWIAUPDISJ-UHFFFAOYSA-L
InChI=1/C12H14N2.2ClH/c1-
13-7-3-11(4-8-13)12-
5-9-14(2)10-6-12;;/
h3-10H,1-2H3;2*1H/
q+2;;/p-2/fC12H14N2.2Cl/
h;2*1h/qm;2*-1
InChI=1/C12H14N2.2ClH/c1-13-7-3-11(4-8-13)12-5-9-14(2)10-6-12;;/h3-10H,1-2H3;2*1H/q+2;;/p-2
Key: FIKAKWIAUPDISJ-NUQVWONBAF
Properties Molecular formula C12H14Cl2N2 Molar mass 257.16 g mol−1 Appearance Off-white powder Density 1.25 g/cm3 Melting point 175-180 °C[1]
Boiling point >300 °C[1]
Solubility in water High Hazards MSDS Oxford MSDS Main hazards Toxic  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Paraquat is the trade name for N,N′-dimethyl-4,4′-bipyridinium dichloride, one of the most widely used herbicides in the world. Paraquat, a viologen, is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic to human beings and animals. Research has shown that it is linked to development of Parkinson's disease.[2]
Contents
Production
Pyridine is coupled with sodium in anhydrous ammonia to give 4,4'-bipyridine, which is then methylated with chloromethane to give the desired compound:[3]
History
Although first synthesized in 1882, paraquat's herbicidal properties were not recognized until 1955.[4] Paraquat was first manufactured and sold by ICI in early 1962, and is today among the most commonly used herbicides.
The European Union approved the use of paraquat in 2004. Subsequently Sweden, supported by Denmark, Austria, and Finland, brought the European Union commission to court. In 2007, the court annulled the directive authorizing paraquat as an active plant protection substance.[5]
Herbicide use
Paraquat is used as a quaternary ammonium herbicide, one of the most widely used herbicides in the world. It is quick-acting, non-selective, and kills green plant tissue on contact. It is redistributed within the plant, but does not harm mature bark. Herbicides are used to protect crops by controlling a wide range of annual and certain perennial weeds that would otherwise reduce compete with the crop for water, nutrients, and light.
The key characteristics that distinguish the non-selective contact herbicide paraquat from other active ingredients used in plant protection products are:
- It is non-selective, which means it kills a wide range of annual grasses and broad-leaved weeds and the tops of established perennial weeds.
- It is very fast-acting.
- It is rain-fast within minutes of application.
- It becomes biologically inactive upon contact with soil.[6]
These properties, especially broad-spectrum weed control and inactivation upon contact with soil, made paraquat an essential tool in the development of no-till farming.[7][8][9]
In the United States, paraquat is available primarily as a liquid in various strengths. It is classified as "restricted use," which means that it can be used only by licensed applicators. In the European Union, paraquat has been forbidden since 2007.[5]
Mode of action
Paraquat inhibits photosynthesis. In light-exposed plants, it accepts electrons from photosystem I (more specifically Fd which is presented with electrons from PS I) and transfers them to molecular oxygen. In this manner, destructive reactive oxygen species are produced. In forming these reactive oxygen species, the oxidized form of paraquat is regenerated, and is again available to shunt electrons from photosystem I to start the cycle again.[10]
Weed resistance management
Problems with herbicide resistant weeds may be addressed by applying herbicides with different modes of action, along with cultural methods such as crop rotation, in integrated weed management (IWM) systems. Paraquat, with its distinctive mode of action, is one of few chemical options that can be used to prevent and mitigate problems with weeds that have become resistant to the very widely used non-selective herbicide glyphosate. [11],[12]
One example is the ‘Double Knock’ system used in Australia.[13] Before planting a crop, weeds are sprayed with glyphosate first, then followed seven to ten days later by a paraquat herbicide. Although twice as expensive as using a single glyphosate spray, the ‘Double Knock’ system is an important resistance management strategy widely relied upon by farmers.[14]
A computer simulation conducted by researchers at the Western Australian Herbicide Research Initiative (WAHRI) calculated that if the herbicide used in land preparation was alternated annually between glyphosate and paraquat, only one field in five would be expected to have glyphosate resistant annual ryegrass (Lolium rigidum) after 30 years, compared to nearly 90% of fields sprayed only with glyphosate.[15] A ‘Double Knock’ regime with paraquat cleaning-up after glyphosate was predicted to keep all fields free of glyphosate resistant ryegrass for at least 30 years.
Scientific use
Paraquat is often used in science to catalyze the formation of reactive oxygen species (ROS), more specifically, the superoxide free radical. Paraquat will undergo redox cycling in vivo, being reduced by an electron donor such as NADPH, before being oxidized by an electron receptor such as dioxygen to produce superoxide, a major ROS.[16]
"Paraquat pot"
During the late 1970s, a controversial program sponsored by the US government sprayed paraquat on marijuana fields in Mexico.[17] Since much of this marijuana was subsequently smoked by Americans, the US government's "Paraquat Pot" program stirred much debate. Perhaps in an attempt to deter people from using marijuana, representatives of the program warned that spraying rendered the crop unsafe to smoke.
However, independent bodies have studied paraquat in this use. Jenny Pronczuk de Garbino,[18] stated: "no lung or other injury in marijuana users has ever been attributed to paraquat contamination". Also a United States Environmental Protection Agency manual states: "... toxic effects caused by this mechanism have been either very rare or nonexistent. Most paraquat that contaminates marijuana is pyrolyzed during smoking to dipyridyl, which is a product of combustion of the leaf material itself (including marijuana) and presents little toxic hazard."[19]
In suicide
A large majority (93%) of fatalities from paraquat poisoning are cases of intentional self-administration, i.e., suicides. In third world countries, paraquat is a "major suicide agent".[20] For instance, in Samoa from 1979–2001, 70% of suicides were by paraquat poisoning. In southern Trinidad from 1996–1997, 76% of suicides were by paraquat.[21]
The reason paraquat is such a widely used suicide agent in third-world countries is due to its widespread availability, low toxic dose (10 ml or 2 teaspoons is enough to kill) and relative low cost. There are campaigns to control or even ban paraquat outright, and there are moves to restrict its availability by requiring user education and the locking up of paraquat stores.
Toxicity
Pure paraquat, when ingested, is highly toxic to mammals, including humans; potentially leading to acute respiratory distress syndrome (ARDS), and there are no specific antidotes. However, fuller's earth or activated charcoal is an effective treatment, if taken in time. Death may occur up to 30 days after ingestion. Diluted paraquat used for spraying is less toxic; thus, the greatest risk of accidental poisoning is during mixing and loading paraquat for use.[4]
In acute toxicity studies using laboratory animals, paraquat has been shown to be highly toxic by the inhalation route and has been placed in Toxicity Category I (the highest of four levels) for acute inhalation effects. However, the EPA has determined that particles used in agricultural practices (400 to 800 μm) are well beyond the respirable range and therefore inhalation toxicity is not a toxicological endpoint of concern. Paraquat is toxic (Category II) by the oral route and moderately toxic (Category III) by the dermal route. Paraquat will cause moderate to severe eye irritation and minimal dermal irritation, and has been placed in Toxicity Categories II and IV (slightly toxic) respectively for these effects.[22]
Even a single swig, immediately spat out, can cause death from fibrous tissue developing in the lungs, leading to asphyxiation.[23]
According to the Center for Disease Control, ingesting paraquat causes symptoms such as liver, lung, heart, and kidney failure within several days to several weeks that can lead to death up to 30 days after ingestion. Those who suffer large exposures are unlikely to survive. Chronic exposure can lead to lung damage, kidney failure, heart failure, and oesophageal strictures.[24] Accidental deaths and suicides from paraquat ingestion are relatively common. For example, there have been 18 deaths in Australia from paraquat poisoning since 2000.[25] Long term exposures to paraquat would most likely cause lung and eye damage, but reproductive/fertility damage was not found by the United States Environmental Protection Agency (EPA) in their review.
Parkinson's disease
Humans
In 2011, a US National Institutes of Health study showed a link between paraquat use and Parkinson's disease in farm workers.[26] A co-author of the paper said that paraquat increases production of certain oxygen derivatives that may harm cellular structures, and that people who used paraquat, or other pesticides with a similar mechanism of action, were more likely to develop Parkinson's disease.[2]
Other mammals
Paraquat-induced toxicity in rats has also been linked to Parkinson's-like neurological degenerative mechanisms.[27] A study by the Buck Institute showed a connection between exposure to paraquat and iron in infancy and mid-life Parkinson's in laboratory mice.[28]
Invertebrates
Paraquat also induces oxidative stress in invertebrates such as Drosophila melanogaster. Paraquat-fed flies suffer early-onset mortality, and significant increases in Superoxide dismutase activity.[29]
References
- ^ a b "Paraquat dichloride". International Programme on Chemical Safety. October 2001. http://www.inchem.org/documents/icsc/icsc/eics0005.htm.
- ^ a b "Two pesticides -- rotenone and paraquat -- linked to Parkinson's disease, study suggests". sciencedaily.com. 2011 [last update]. http://www.sciencedaily.com/releases/2011/02/110214115442.htm. Retrieved October 25, 2011.
- ^ "Paraquat and Diquat". IPCS INCHEM. http://www.inchem.org/documents/ehc/ehc/ehc39.htm.
- ^ a b "Paraquat". Pesticides News 32: 20–21. 1996.
- ^ a b COURT OF FIRST INSTANCE OF THE EUROPEAN COMMUNITIES, PRESS RELEASE No° 45/07
- ^ Revkin, A. C. (1983). "Paraquat: A potent weed killer is killing people". Science Digest 91 (6): 36–38.
- ^ Hood A. E. M., Jameson H. R. and Cotterell R. (1963). Destruction of pastures by paraquat as a substitute for ploughing. Nature, 197, 4869, 381
- ^ Hood A. E. M. (1965). Ploughless farming using “Gramoxone”. Outlook on Agriculture IV, 6, 286-294
- ^ Huggins D R & Reganold J. P. (2008). No-Till: the Quiet Revolution. Scientific American, July 2008, pp 70-77
- ^ Summers L.A. (1980) The Bipyridinium Herbicides. Academic Press, New York, NY.
- ^ Beckie H. J. (2011). Herbicide-resistant weed-management: focus on glyphosate. Pest Management Science, 67, 1037-1048
- ^ Eubank T. W. et al. (2008). Glyphosate-resistant horseweed (Conyza canadensis) control using glyphosate-, paraquat-, and glufosinate-based herbicide programs. Weed Technology, 22, (1), 16-21
- ^ Borger C.P. & Hashem A. (2007). Evaluating the double knockdown technique: sequence, application interval, and annual ryegrass growth stage. Australian Journal of Agricultural Research, 58, 265-271
- ^ Walsh M.J. & Powles S.B. (2007). Management strategies for herbicide resistant weed populations in Australian dryland crop production systems. Weed Technology, 12, 332-338
- ^ Neve, P et al. (2003). Simulating evolution of glyphosate resistance. II. Past, present and future use glyphosate use in Australian cropping. Weed Research, 43, 418-427
- ^ Bus et al. (1984). "Paraquat: model for oxidant-initiated toxicity". Environmental Health Perspectives 55: 37–46.
- ^ Panic over Paraquat, Time Magazine, May 1, 1978
- ^ Pronczuk de Garbino J, Epidemiology of paraquat poisoning, in: Bismuth C, and Hall AH (eds), Paraquat Poisoning: Mechanisms, Prevention, Treatment, pp. 37-51, New York: Marcel Dekker, 1995.
- ^ Reigart, J. Routt and Roberts, James R. Recognition and Management of Pesticide Poisonings, 5th edition. Washington, DC: United States Environmental Protection Agency, 1999. Book available online
- ^ Dinham, B. (1996). "Active Ingredient fact sheet, Paraquat". Pesticide News 32: 20–1.
- ^ Paraquat and Suicide, Pestizid Aktions-Netzwerk e.V. (PAN Germany).
- ^ Paraquat Dichloride, United States Environmental Protection Agency, accessed 16 August 2007.
- ^ Buzik, Shirley C.; Schiefer, H. Bruno; Irvine, Donald G. (1997). Understanding Toxicology: Chemicals, Their Benefits and Risks. Boca Raton: CRC Press. p. 31. ISBN 0-8493-2686-9.
- ^ Center for Disease Control, Facts about Paraquat, accessed 13 October 2006.
- ^ "Poisoned Latrobe," Gary Stevens, Valley Express Feb. 8, 2008.
- ^ Tanner, Caroline M.; Freya Kamel, G. Webster Ross, Jane A. Hoppin, Samuel M. Goldman, Monica Korell, Connie Marras, Grace S. Bhudhikanok, Meike Kasten, Anabel R. Chade, Kathleen Comyns, Marie Barber Richards, Cheryl Meng, Benjamin Priestley, Hubert H. Fernandez, Franca Cambi, David M. Umbach, Aaron Blair, Dale P. Sandler, J. William Langston (2011). "Rotenone, Paraquat and Parkinson’s Disease". Environmental Health Perspectives. doi:10.1289/ehp.1002839. ISSN 0091-6765. PMID 21269927. http://ehp03.niehs.nih.gov/article/fetchArticle.action?articleURI=info%3Adoi%2F10.1289%2Fehp.1002839. Retrieved 2011-02-14.
- ^ K. Ossowska, M. S'Mialowska, K. Kuter, J. Wieron'ska, B. Zieba, J. Wardas, P. Nowak, J. Dabrowska, A. Bortel, I. Biedka, G. Schulze and H. Rommelspacher (2006). "Degeneration of dopaminergic mesocortical neurons and activation of compensatory processes induced by a long-term paraquat administration in rats: Implications for Parkinson's disease". Neuroscience 141 (4): 2155–2165. doi:10.1016/j.neuroscience.2006.05.039. PMID 16797138.
- ^ "Combined Exposure to Environmental Toxics Accelerates Age-related Development of Parkinson's Disease in Mice" (Press release). Buck Institute for Aging Research. June 2007. http://www.buckinstitute.org/buck-news/combined-exposure-environmental-toxics-accelerates-age-related-development-parkinsons-diseas.
- ^ T.Z. Rzezniczak, L.A. Douglas, J.H. Watterson, and T.J.S. Merritt (2011). "Paraquat administration in Drosophila for use in metabolic studies of oxidative stress". Analytical Biochemistry (journal) 419 (2): 345–347. doi:10.1016/j.ab.2011.08.023. PMID 21910964.
Further reading
- Slade, P. (1966). "The Fate of Paraquat Applied to Plants". Weed Research 6 (2): 158. doi:10.1111/j.1365-3180.1966.tb00876.x.
- Smith, S. N.; Lyon, A. J. E.; Sahid, Ismail BIN (1976). "The Breakdown of Paraquat and Diquat by Soil Fungi". New Phytologist 77 (3): 735. doi:10.1111/j.1469-8137.1976.tb04668.x.
External links
- "Paradox Product". Sinon do Brasil. http://www.sinon.com.br/english/products_paradox.php.
- "Stop Paraquat". The Berne Declaration. http://www.evb.ch/en/f25000087.html.
- "The Paraquat Information Center". Syngenta Crop Protection AG. http://www.paraquat.com.
Pest control: herbicides Anilides/Anilines acetochlor · alachlor · asulam · butachlor · diethatyl · diflufenican · dimethenamid · flamprop · metazachlor · metolachlor · pendimethalin · pretilachlor · propachlor · propanil · trifluralinAromatic acids Arsenicals Organophosphorus Phenoxy Pyridines Quaternary Triazines ametryn · atrazine · cyanazine · hexazinone · prometon · prometryn · propazine · simazine · simetryn · terbuthylazine · terbutrynUreas Others 3-AT · bromoxynil · clomazone · DCBN · dinoseb · juglone · mesotrione · methazole · metham sodium · sulfentrazoneCategories:- Herbicides
- Pyridines
Wikimedia Foundation. 2010.
