- Organic acid
-
Acids and Bases Acid dissociation constant
Acid-base extraction
Acid–base reaction
Acid–base titration
Dissociation constant
Acidity function
Buffer solutions
pH
Proton affinity
Self-ionization of waterAcid types Brønsted · Lewis · Mineral
Organic · Strong
Superacids · WeakBase types Brønsted · Lewis · Organic
Strong · Superbases
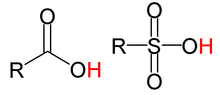
Non-nucleophilic · WeakAn organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. The relative stability of the conjugate base of the acid determines its acidity. Other groups can also confer acidity, usually weakly: –OH, –SH, the enol group, and the phenol group. In biological systems, organic compounds containing these groups are generally referred to as organic acids.
A few common examples include:
Contents
Characteristics
In general, organic acids are weak acids and do not dissociate completely in water, whereas the strong mineral acids do. Lower molecular mass organic acids such as formic and lactic acids are miscible in water, but higher molecular mass organic acids, such as benzoic acid, are insoluble in molecular (neutral) form.
On the other hand, most organic acids are very soluble in organic solvents. p-Toluenesulfonic acid is a comparatively strong acid used in organic chemistry often because it is able to dissolve in the organic reaction solvent.
Exceptions to these solubility characteristics exist in the presence of other substituents that affect the polarity of the compound.
Applications
Simple organic acids like formic or acetic acids are used for oil and gas well stimulation treatments. These organic acids are much less reactive with metals than are strong mineral acids like hydrochloric acid (HCl) or mixtures of HCl and hydrofluoric acid (HF). For this reason, organic acids are used at high temperatures or when long contact times between acid and pipe are needed.[citation needed]
The conjugate bases of organic acids such as citrate and lactate are often used in biologically-compatible buffer solutions.
Citric and oxalic acids are used as rust removal. As acids, they can dissolve the iron oxides, but without damaging the base metal as do stronger mineral acids. In the dissociated form, they may be able to chelate the metal ions, helping to speed removal.[citation needed]
Biological systems create many and more complex organic acids such as L-lactic, citric, and D-glucuronic acids that contain hydroxyl or carboxyl groups. Human blood and urine contain these plus organic acid degradation products of amino acids, neurotransmitters, and intestinal bacterial action on food components. Examples of these categories are alpha-ketoisocaproic, vanilmandelic, and D-lactic acids, derived from catabolism of L-leucine and epinephrine (adrenaline) by human tissues and catabolism of dietary carbohydrate by intestinal bacteria, respectively.
The general structure of a few organic acids. From left to right: carboxylic acid, sulfonic acid. The acidic hydrogen in each molecule is colored red.
Application in food
Organic acids are used in food preservation because of their effects on bacteria. The key basic principle on the mode of action of organic acids on bacteria is that non-dissociated (non-ionized) organic acids can penetrate the bacteria cell wall and disrupt the normal physiology of certain types of bacteria that we call pH-sensitive, meaning that they cannot tolerate a wide internal and external pH gradient. Among those bacteria are Escherichia coli, Salmonella spp., C. perfringens, Listeria monocytogenes, and Campylobacter species.
Upon passive diffusion of organic acids into the bacteria, where the pH is near or above neutrality, the acids will dissociate and lower the bacteria internal pH, leading to situations that will impair or stop the growth of bacteria. On the other hand, the anionic part of the organic acids that cannot escape the bacteria in its dissociated form will accumulate within the bacteria and disrupt many metabolic functions, leading to osmotic pressure increase, incompatible with the survival of the bacteria.
It has been well demonstrated that the state of the organic acids (undissociated or dissociated) is extremely important to define their capacity to inhibit the growth of bacteria, compared to undissociated acids.
Lactic acid and its salts sodium lactate and potassium lactate are widely used as antimicrobials in food products, in particular, meat and poultry such as ham and sausages.[1]
Application in nutrition and animal feeds
Organic acids have been used successfully in pig production for more than 25 years. Although less research has been done in poultry, organic acids have also been found to be effective in poultry production.
Organic acids (C1–C7) are widely distributed in nature as normal constituents of plants or animal tissues. They are also formed through microbial fermentation of carbohydrates mainly in the large intestine. They are sometimes found in their sodium, potassium, or calcium salts, or even stronger double salts.
Organic acids added to feeds should be protected to avoid their dissociation in the crop and in the intestine (high pH segments) and reach far into the gastrointestinal tract, where the bulk of the bacteria population is located.
From the use of organic acids in poultry and pigs, one can expect an improvement in performance similar to or better than that of antibiotic growth promoters, without the public health concern, a preventive effect on the intestinal problems like necrotic enteritis in chickens and Escherichia coli infection in young pigs. Also one can expect a reduction of the carrier state for Salmonella species and Campylobacter species.
See also
References
- ^ Applications for lactic acid.http://www.purac.com/purac_com/67cbf5490d83dc478dafbd96cab841b1.php
Further reading
- Dibner, J. J.; Butin, P. (2002). "Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism". J. Appl. Poultry Res. 11 (4): 453–463. http://japr.fass.org/cgi/content/abstract/11/4/453.
- Patanen, K. H.; Mroz, Z. (1999). "Organic acids for preservation". In Block, S. S.. Disinfection, sterilization & preservation (5th ed.). Philadelphia: Lea Febiger. ISBN 0683307401.
- Brul, S.; Coote, P. (1999). "Preservative agents in foods, mode of action and microbial resistance mechnismes". Intl. J. Food Microbiology 50 (1–2): 1–17. PMID 10488839.
Categories:- Organic acids
Wikimedia Foundation. 2010.