- 2,4-Dichlorophenoxyacetic acid
-
2,4-Dichlorophenoxyacetic acid 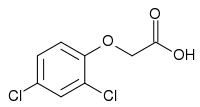 (2,4-dichlorophenoxy)acetic acidOther names2,4-D
(2,4-dichlorophenoxy)acetic acidOther names2,4-D
hedonal
trinoxolIdentifiers CAS number 94-75-7 
PubChem 1486 ChemSpider 1441 
KEGG C03664 
ChEBI CHEBI:28854 
ChEMBL CHEMBL367623 
Jmol-3D images Image 1 - Clc1cc(Cl)ccc1OCC(=O)O
Properties Molecular formula C8H6Cl2O3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C, 414 K, 285 °F
Boiling point 160 °C, 433 K, 320 °F (0.4 mm Hg)
Solubility in water 900 mg/L Related compounds Related compounds 2,4,5-T, Dichlorprop  acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic pesticide/herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is a synthetic auxin (plant hormone), and as such it is often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. It was a major ingredient in Agent Orange.
Contents
History
2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[2] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crops, because it only kills dicots (broadleaf plants), leaving behind monocots (grasses).
Genetically modified crops
Dow has demonstrated maize and soybean resistance to 2,4-D due to insertion of a bacterial aryloxyalkanoate dioxygenase gene.[3] This is intended as an alternative to Roundup Ready crops due to the increasing prevalence of glyphosate resistant weeds.
Mechanism of action
2,4-D is a synthetic auxin, which is a class of plant hormones. It is absorbed through the leaves and is translocated to the meristems of the plant. Uncontrolled, unsustainable growth ensues, causing stem curl-over, leaf withering, and eventual plant death. 2,4-D is typically applied as an amine salt, but more potent ester versions exist as well.
Manufacture
2,4-D is a member of the phenoxy family of herbicides, which include:
- 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T)
- 2-Methyl-4-chlorophenoxyacetic acid (MCPA)
- 2-(2-Methyl-4-chlorophenoxy)propionic acids (mecoprop, MCPP)
- 2-(2,4-Dichlorophenoxy)propionic acid (dichlorprop, 2,4-DP)
- (2,4-Dichlorophenoxy)butyric acid (2,4-DB)
2,4-D is manufactured from chloroacetic acid and 2,4-dichlorophenol, which is itself produced by chlorination of phenol. Alternatively, it may be produced by the chlorination of phenoxyacetic acid. The production processes creates several contaminants including isomers, monochlorophenol, and other polychlorophenols and their acids.[4]
Dioxin impurities
Some preparations of 2,4-D are contaminated with dioxins due to the manufacturing process.[5] Tetrachlorodibenzo-p-dioxin (TCDD) is classified as "carcinogenic to humans" by International Agency for Research on Cancer.[6]
Contamination is predominantly of the type with 2 or 3 chlorine atoms. Another form of dioxin, 2,7-dichlorodibenzo-p-dioxin (DCDD), an inevitable by-product of 2,4-D manufacturing,[citation needed] was found to be equipotent to dioxin TCDD in its toxic effect on the immunity of mice. TCDD received all the publicity while the DCDD component was largely forgotten. To this day, DCDD is not regulated or monitored by the EPA and PMRA, even though DCDD levels could be at much higher levels than TCDD.[citation needed] The typical smell of 2,4-D is the break-down product 2,4-dichlorophenol, which is a suspected endocrine disrupter and possible carcinogen.[citation needed] 2,4-D is toxic to the liver at small dosages. Increases in liver function tests, jaundice, acute hepatitis, lobular and portal inflammation indicative of a toxic reaction, as well as permanent damage leading to cirrhosis in exposed golfers [7][8][9]
The defoliant and herbicide Agent Orange, used extensively throughout the Vietnam War, contained 2,4-D. The controversies associated with the use of Agent Orange were associated with a contaminant (dioxin) in the 2,4,5-T component.[10]
Applications
2,4-D is primarily used as an herbicide.[11] It is sold in various formulations under a wide variety of brand names. 2,4-D can be found in lawn herbicide mixtures such as "Weed B Gon MAX", "PAR III", "Trillion", "Tri-Kil", "Killex" and "Weedaway Premium 3-Way XP Turf Herbicide". All of these mixtures typically contain three active ingredients: 2,4-D, mecoprop and dicamba. Over 1,500 herbicide products contain 2,4-D as an active ingredient.[citation needed]
2,4-D is most commonly used for:
- Weed control in lawns and other turf
- No-till burndown
- Control of weeds and brush along fences and highway and railroad rights of way
- Conifer release (control of broad-leaf trees in conifer plantings)
- Grass hayfields and pastures
- Cereal grains
- Corn and sorghum (occasionally)
- As a synthetic auxin analog
2,4-D continues to be used, where legal, for its low cost. However, where municipal lawn pesticide bylaws exist, such as in Canada,[12] alternatives such as corn gluten meal and vinegar-based products are increasingly being used to combat weeds.
Toxicity
Cancer risk
Different organizations have taken different stances on cancer risk of 2,4-D. On August 8, 2007, the United States Environmental Protection Agency issued a ruling that stated that existing data does not support a conclusion that links human cancer to 2,4-D exposure.[13] The International Agency for Research on Cancer (IARC) has classified 2,4-D among the phenoxy acid herbicides MCPA and 2,4,5-T as a class 2B carcinogen - possibly carcinogenic to humans.[14] A 1995 panel of 13 scientists reviewing studies on the carcinogenicity of 2,4-D had divided opinions, but the predominant opinion was that it is possible that 2,4-D causes cancer in humans.[15]
A 1990 study of farmers in Nebraska, even when adjusting for exposure to other chemicals, found that 2,4-D exposure substantially increased the risk of Non-Hodgkin's lymphoma (NHL).[16] A 2000 study of 1517 former employees of Dow Chemical Company who had been exposed to the chemical in manufacturing or formulating 2,4-D found no significant increase in risk of mortality due to NHL following 2,4-D exposure, but did find an increase in risk of mortality due to amyotrophic lateral sclerosis.[17]
Other
The LD50 determined in an acute toxicity rat study is 639 mg/kg.[18] Single oral doses of 5 and 30 mg/kg body weight did not cause any acute toxic effects in human volunteers. This chemical has been associated with the risk of amyotrophic lateral sclerosis.[19]
The amine salt formulations can cause eye damage (blindness) on contact; ester formulations are considered non-irritating to the eyes.
One study found that occupational exposure to 2,4-D caused male reproductive problems, including dead and malformed sperm.[20]
Concerns regarding neurotoxicity have been voiced with increased sensitivity to amphetamine and thus concerns of increased risk of drug addiction among those exposed.[21]
Treatment
While urinary alkalinisation has been used in acute poisonings, evidence to support its use is poor.[22]
Environmental behavior
Owing to the longevity and extent of use, 2,4-D is among the most thoroughly studied herbicides with respect to environmental properties. 2,4-D applied at 1.16 lb/acre to bluegrass turf in a laboratory experiment had a half-life of ten days. Other studies found half-life figures between 1.5 and 16 days. Soil microbes are primarily responsible for its disappearance in soil. Studies in Alaska and Canada failed to detect leaching in 22 weeks or from spring to fall,[23] but 2,4-D has been included on the EPA list of compounds that are likely to leach from soil.
In aquatic environments microorganisms readily degrade 2,4-D and breakdown by sunlight is not a major reason for loss. Rates of breakdown increase with increased nutrients, sediment load and dissolved organic carbon. Under oxygenated conditions the half-life can be short, in the order of one week to several weeks. 2,4-D interferes with normal plant growth processes. Uptake of the compound is through leaves, stems and roots; however, it is, in general, nonpersistent. In one study when 2,4-D was applied to grass, there were 80 ppm at day zero, 45 ppm at 14 days, and 6 ppm at 56 days. Breakdown in plants is by a variety of biological and chemical pathways.[24]
A number of 2,4-D-degrading bacteria have been isolated and characterized from a variety of environmental habitats.[25][26] Metabolic pathways for the compound’s degradation have been available for many years, and genes encoding 2,4-D catabolism have been identified for several organisms. As a result of the extensive metadata on environmental behavior, physiology and genetics, 2,4-D was the first herbicide for which the bacteria actively responsible for in situ degradation was demonstrated.[27] This was accomplished using the technique of DNA-based stable isotope probing, which enables a microbial function (activity), such as degrading a chemical, to be linked with the organism’s identity without the need to culture the organism involved.[28] This advancement has been particularly beneficial for the study of soil microorganisms, since only a very small fraction of the thousands of species present in highly diverse soil bacterial communities can be isolated in pure culture.
Despite its short half-life in soil and in aquatic environments, the compound has been detected in groundwater supplies in at least five States and in Canada.[29] It has also been detected in surface waters throughout the United States at very low concentrations.
Legal issues
2,4-D has been evaluated by the European Union and included on its list of approved herbicides, stating inter alia that "the review [of 2,4-D] has established that the residues arising from the proposed uses, consequent on application consistent with good plant protection practice, have no harmful effects on human or animal health."[30] Concern over 2,4-D is such that it is currently not approved for use on lawns and gardens in Sweden,[31] Denmark, Norway, Kuwait and the Canadian provinces of Québec [32] and Ontario.[33] 2,4-D use is severely restricted in the country of Belize. In 2005, the United States Environmental Protection Agency approved the continued use of 2,4-D.[34] In Canada, the Pest Management Regulatory Agency (PMRA) has placed a condition of registration on 2,4-D such that the 2,4-D registrant(s) must provide the PMRA with a required developmental neurotoxicity study by September 20, 2009.[35] According to the PMRA, the due date of the study has since been extended to early 2010.
References
- ^ 24d.org Industry Task Force II on 2,4-D Research Data
- ^ See the review article: J. H. Quastel, "2,4-dichlorophenoxyacetic acid (2,4-D) as a selective herbicide," Agricultural Control Chemicals (Washington, D.C.: American Chemical Society, 1950), Chapter 45, pages 244-249.
- ^ Wright, T. R.; Shan, G.; Walsh, T. A.; Lira, J. M.; Cui, C.; Song, P.; Zhuang, M.; Arnold, N. L. et al. (2010). "Robust crop resistance to broadleaf and grass herbicides provided by aryloxyalkanoate dioxygenase transgenes". Proceedings of the National Academy of Sciences 107 (47): 20240. doi:10.1073/pnas.1013154107.
- ^ "2,4-Dichlorophenoxyacetic Acid (2,4-D)". International Programme on Chemical Safety. http://www.inchem.org/documents/ehc/ehc/ehc29.htm.
- ^ "Dichlorophenoxyacetic acid, 2,4- (2,4-D): environmental aspects (EHC 84, 1989)". United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. http://www.inchem.org/documents/ehc/ehc/ehc84.htm.
- ^ Hardell L, Walker MJ, Walhjalt B, Friedman LS, Richter ED (March 2007). "Secret ties to industry and conflicting interests in cancer research". Am. J. Ind. Med. 50 (3): 227–33. doi:10.1002/ajim.20357. PMID 17086516.
- ^ Leonard C, Burke CM, O'Keane C, Doyle JS (May 1997). ""Golf ball liver": agent orange hepatitis". Gut 40 (5): 687–8. PMC 1027176. PMID 9203952. http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=9203952.
- ^ Johnston S, McCusker G, Tobinson TJ (January 1998). ""Golf ball liver": a cause of chronic hepatitis ?". Gut 42 (1): 143. doi:10.1136/gut.42.1.143a. PMC 1726975. PMID 9505901. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1726975.
- ^ 2,4-dichlorophenol - toxicity, ecologicial toxicity and regulatory information
- ^ The Monsanto Investigation
- ^ Record in the Household Products Database of NLM
- ^ Private Property Pesticide By-laws In Canada
- ^ EPA: Federal Register: 2,4-D, 2,4-DP, and 2,4-DB; Decision Not to Initiate Special Review
- ^ IARC monographs on the evaluation of carcinogenic risks to humans: An updating of IARC Monographs volumes 1 to 42. Supplement 7, WHO, Lyon, France 1987.
- ^ M. A. Ibrahim, G. G. Bond, T. A. Burke, P. Cole, F. N. Dost, P. E. Enterline, M. Gough, R. S. Greenberg, W. E. Halperin, E. McConnell, I. C. Munro, J. A. Swenberg, S. H. Zahm, and J. D. Graham, "Weight of the evidence on the human carcinogenicity of 2,4-D", Environ Health Perspect., 1991 December; 96: 213–222.
- ^ Zahm, Shelia Hoar; Weisenburger, Dennis D.; Babbitt, Paula A.; Saal, Robert C.; Vaught, Jimmie B.; Cantor, Kenneth P.; Blair, Aaron, "A Case-Control Study of Non-Hodgkin's Lymphoma and the Herbicide 2,4-Dichlorophenoxyacetic Acid (2, 4-D) in Eastern Nebraska", Epidemiology, Vol. 1, No. 5., Sep. 1990.
- ^ "C J Burnsa, K K Beardb, J B Cartmill, "Mortality in chemical workers potentially exposed to 2,4-dichlorophenoxyacetic acid (2,4-D) 1945-94: an update", Occupational and Environmental Medicine, Vol. 58, pp. 24-30, 2001.". Archived from the original on 2009-05-14. http://oem.bmj.com/cgi/content/abstract/58/1/24. Retrieved 2009-05-08.
- ^ US EPA 2,4-D Reregistration Eligiblity Decision, 2006
- ^ Burns CJ, Beard KK, Cartmill JB (January 2001). "Mortality in chemical workers potentially exposed to 2,4-dichlorophenoxyacetic acid (2,4-D) 1945-94: an update". Occup Environ Med 58 (1): 24–30. doi:10.1136/oem.58.1.24. PMC 1740039. PMID 11119631. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1740039.
- ^ Lerda, D; Rizzi, R (1991). "Study of reproductive function in persons occupationally exposed to 2,4-dichlorophenoxyacetic acid (2,4-D)". Mutation research 262 (1): 47–50. PMID 1986284.
- ^ Jones DC, Miller GW (September 2008). "The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction". Biochem. Pharmacol. 76 (5): 569–81. doi:10.1016/j.bcp.2008.05.010. PMID 18555207.
- ^ Roberts DM, Buckley NA (2007). Roberts, Darren M. ed. "Urinary alkalinisation for acute chlorophenoxy herbicide poisoning". Cochrane Database Syst Rev (1): CD005488. doi:10.1002/14651858.CD005488.pub2. PMID 17253558.
- ^ Forest Service, (1984). Pesticide Background Statements, Vol. I Herbicides. United States Department of Agriculture, Agriculture Handbook No. 633.
- ^ National Research Council Canada (1978). Phenoxy Herbicides - Their Effects on Environmental Quality with Accompanying Scientific Criteria for 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD). Subcommittee on Pesticides and Related Compounds, NRC Associate Committee on Scientific Criteria for Environmental Quality, Ottawa, Canada.
- ^ Cavalca, L., A. Hartmann, N. Rouard, and G. Soulas. 1999. Diversity of tfdC genes: distribution and polymorphism among 2,4-dichlorophenoxyacetic acid degrading soil bacteria. FEMS Microbiology Ecology 29: 45-58.
- ^ Suwa, Y., A.D. Wright, F. Fukimori, K.A. Nummy, R.P. Hausinger, W.E. Holben, and L.J. Forney. 1996. Characterization of a chromosomally encoded 2,4-dichlorophenoxyacetic acid alpha-ketoglutafate dioxygenase from Burkholderia sp. strain RASC. Applied and Environmental Microbiology 62: 2464-2469.
- ^ Cupples, A.M. and G.K. Sims. 2007. Identification of In Situ 2,4-Dichlorophenoxyacetic Acid-Degrading Soil Microorganisms using DNA-Stable Isotope Probing. Soil Biology and Biochemistry 39: 232-238.
- ^ Radajewski, S., P. Ineson, N.R. Parekh, and J.C. Murrell. 2000. Stable-isotope probing as a tool in microbial ecology. Nature 403: 646-649.
- ^ Howard, Philip H. Handbook of Environmental Fate and Exposure Data for Organic Chemicals. Lewis Publishers Chelsea, Michigan.
- ^ EUROPA - Plant Health - Plant Protection - Evaluation & Authorisation - Existing active substances - Reports
- ^ http://sv.wikipedia.org/wiki/2,4-diklorfenoxiättiksyra
- ^ http://www.mddep.gouv.qc.ca/pesticides/permis-en/code-gestion-en/espace-vert.htm
- ^ http://www.ene.gov.on.ca/en/news/2009/030401.php
- ^ 2,4-D (2,4-dichlorophenoxyacetic acid) | Reregistration | Regulating Pesticides | Pesticides | US EPA
- ^ Proposed Acceptability for Continuing Registration PACR2007-06
External links
Government and academic references:
- 2,4-D Technical Fact Sheet - National Pesticide Information Center
- 2,4-D Pesticide Information Profile - Extension Toxicology Network
- EPA 2,4-D Reregistration Eligibility Decision
- 2,4-D RED Facts
- Biomonitoring Data for 2,4-Dichlorophenoxyacetic Acid in the US and Canada: Interpretation in a Public Health Risk Assessment Context Using Biomonitoring Equivalents, Environmental Health Perspectives online 12. 8. 2009
- Burns et al.: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1740039/?tool=pubmed
Industry website:
Health and environmental references:
- Highlights of Major Problems with PMRA's Feb. 21, 2005 Review on 2,4-D Herbicide
- Overview of the toxic effects of 2,4-D
- 2,4-D: The Wrong Symbol for Pesticides
Pest control: herbicides Anilides/Anilines acetochlor · alachlor · asulam · butachlor · diethatyl · diflufenican · dimethenamid · flamprop · metazachlor · metolachlor · pendimethalin · pretilachlor · propachlor · propanil · trifluralinAromatic acids Arsenicals Organophosphorus Phenoxy Pyridines Quaternary Triazines ametryn · atrazine · cyanazine · hexazinone · prometon · prometryn · propazine · simazine · simetryn · terbuthylazine · terbutrynUreas Others 3-AT · bromoxynil · clomazone · DCBN · dinoseb · juglone · mesotrione · methazole · metham sodium · sulfentrazoneCategories:- Organochlorides
- Herbicides
- Auxins
- Phenol ethers
- Acetic acids
Wikimedia Foundation. 2010.