- Dehydroascorbic acid
-
Dehydroascorbic acid 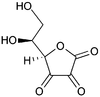 (5R)-5-[(1S)-1,2-dihydroxyethyl]furan-2,3,4(5H)-trione
(5R)-5-[(1S)-1,2-dihydroxyethyl]furan-2,3,4(5H)-trioneIdentifiers CAS number 490-83-5 
PubChem 440667 ChemSpider 389547 
ChEBI CHEBI:27956 
Jmol-3D images Image 1 - O=C1C(=O)C(=O)O[C@@H]1[C@@H](O)CO
Properties Molecular formula C6H6O6 Molar mass 174.11 g mol−1  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dehydroascorbic acid (DHA) is an oxidized form of ascorbic acid. It is actively imported into the endoplasmic reticulum of cells via glucose transporters. It is trapped therein by reduction back to ascorbate by glutathione and other thiols.[1] Therefore, L-dehydroascorbic acid is a vitamin C compound much like L-ascorbic acid. The (free) chemical radical semidehydroascorbic acid (SDA) also belongs to the group of oxidized ascorbic acids.
Contents
Structure and Physiology
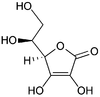
 Top: ascorbic acid
Top: ascorbic acid
(reduced form of vitamin C)
Bottom: dehydroascorbic acid
(nominal oxidized form of vitamin C)Although there exists a sodium-dependent transporter for vitamin C, it is mainly present in specialized cells, whereas the glucose transporters, most notably GLUT1, ensure in most cells of the body the transport of vitamin C (in its oxidized form, DHA)[2] where recycling back to ascorbate generates the necessary enzyme cofactor and intracellular antioxidant, (see Transport to mitochondria).
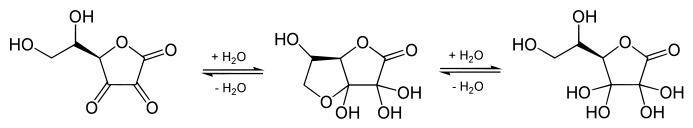
The structure shown here for DHA is the commonly shown textbook structure. This 1,2,3-tricarbonyl is too electrophilic to survive more than a few milliseconds in aqueous solution, however. The actual structure shown by spectroscopic studies is the result of rapid hemiacetal formation between the 6-OH and the 3-carbonyl groups. Hydration of the 2-carbonyl is also observed.[3] The lifetime of the stabilized species is commonly said to be about 6 minutes under biological conditions.[4] Destruction results from irreversible hydrolysis of the ester bond, with additional degradation reactions following.[5] Crystallization of solutions of DHA gives a pentacyclic dimer structure of indefinite stability. Recycling of ascorbate via active transport of DHA into cells, followed by reduction and reuse, mitigates the inability of humans to synthesize it from glucose.[6]
Transport to mitochondria
Vitamin C accumulates in mitochondria, where most of the free radicals are produced, by entering as DHA through the glucose transporters, GLUT1. Ascorbic acid protects the mitochondrial genome and membrane.[2]
Transport to the brain
Vitamin C does not pass from the blood stream into the brain, although the brain is one of the organs which has the greatest concentration of vitamin C. Instead, DHA is transported through the blood-brain barrier via GLUT1 transporters, and then converted back to ascorbate.[7]
Use
As a dietary supplement, dehydroascorbic acid is found in some vitamin C tablets.[8]
As a cosmetic ingredient, dehydroascorbic acid is used to enhance the appearance of the skin.[9] It may be used in a process for permanent waving of hair[10] and in a process for sunless tanning of skin.[11]
In a cell culture growth medium, dehydroascorbic acid has been used to assure the uptake of vitamin C into cell types that do not contain ascorbic acid transporters. However that same study also indicated that unlike ascorbic acid (regular vitamin C), dehydroscorbic acid may promote rather than discourage cancer cell growth as one of it's experimental groups had increased cancer growth after dehydroscorbic acid treatment, but is the only known study with that outcome and the other dehydroscorbic groups in the study did not show that same effect.[12]
As a pharmaceutical agent, some research has suggested that administration of dehydroascorbic acid may confer protection from neuronal injury following an ischemic stroke.[7] The literature contains many reports on the antiviral effects of vitamin C,[13] and one study suggests dehydroascorbic acid has stronger antiviral effects and a different mechanism of action than ascorbic acid.[14]
References
- ^ Welch, R.W.; Wang, Y.; Crossman, A., Jr.; Park, J.B.; Kirk, K.L.; Levine, M.; "Accumulation of Vitamin C (Ascorbate) and Its Oxidized Metabolite Dehydroascorbic Acid Occurs by Separate Mechanisms," J. Biol. Chem. 1995 270 12584-92.
- ^ a b KC S, Carcamo JM, Golde DW (2005). "Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury". FASEB J 19 (12): 1657–67. doi:10.1096/fj.05-4107com. PMID 16195374. http://www.fasebj.org/cgi/content/full/19/12/1657.
- ^ Kerber, R.C.; "'As Simple as Possible, but not Simpler' -- The Case of Dehydroascorbic Acid," J. Chem. Ed. 85 (2007) 1541-5.
- ^ May, J.M.; "Ascorbate Function and Metabolism in the Human Erythrocyte," Frontiers in Bioscience, 3 (1981) d1-10.
- ^ Kimoto, E.; Tanaka, H.; Ohmoto, T.; Choami, M.; "Analysis of the Transformation Products of Dehydro-L-Ascorbic Acid by Ion-Pairing High-Performance Liquid Chromatography," Anal. Biochem. 214 (1993) 38-44.
- ^ Montel-Hagen, A.; Kinet, S.; Manel, N.; Mongellaz, C.; Prohaska,R.; Battini, J.L.; Delaunay,J.; Sitbon, M.; Taylor, N.; "Erythrocyte GLUT1 triggers Dehydroascorbic Acid Uptake in Mammals unable to Synthesize Vitamin C," Cell 132 (2008) 1039-48.
- ^ a b Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, Fox WD, Israel RJ, Boyd TA, Golde DW, Connolly ES Jr. (2001). "Dehydroascorbic acid, a blood–brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke". Proceedings of the National Academy of Sciences 98 (20): 11720–11724. doi:10.1073/pnas.171325998. PMC 58796. PMID 11573006. http://www.pnas.org/cgi/content/full/98/20/11720.
- ^ Higdon, Jane (May, 2001). "The Bioavailability of Different Forms of Vitamin C". The Linus Pauling Institute. http://lpi.oregonstate.edu/ss01/bioavailability.html. Retrieved 2010-11-10.
- ^ "ReCverin". http://recverin.com. Retrieved 2010-11-10.
- ^ US Patent 6,506,373 (issued Jan. 14, 2003)
- ^ U.S. Patent Application No. 10/685,073 Publication No. 20100221203 (published Sept. 2, 2010)
- ^ Heaney ML, Gardner JR, Karasavvas N, Golde DW, Scheinberg DA, Smith EA, O'Conner OA (2008). "Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs". Cancer Research 68 (19): 8031–8038. doi:10.1158/0008-5472.CAN-08-1490. PMID 18829561. http://cancerres.aacrjournals.org/content/68/19/8031.full.
- ^ Jariwalla, R.J. & Harakeh S. (1997). Mechanisms underlying the action of vitamin C in viral and immunodeficiency disease. In L. Packer & J. Fuchs (Eds.), Vitamin C in health and disease (pp. 309-322). New York:Marcell Dekker, Inc.
Further reading
- Nualart F, Rivas C, Montecinos V, Godoy A, Guaiquil V, Golde D, Vera J (2003). "Recycling of vitamin C by a bystander effect". J Biol Chem 278 (12): 10128–33. doi:10.1074/jbc.M210686200. PMID 12435736. http://www.jbc.org/cgi/content/full/278/12/10128.
Vitamins (A11) Fat soluble D2 (Ergosterol, Ergocalciferol#) · D3 (7-Dehydrocholesterol, Previtamin D3, Cholecalciferol, 25-hydroxycholecalciferol, Calcitriol (1,25-dihydroxycholecalciferol), Calcitroic acid) · D4 (Dihydroergocalciferol) · D5 · D analogues (Dihydrotachysterol, Calcipotriol, Tacalcitol, Paricalcitol)Water soluble B1 (Thiamine#) · B2 (Riboflavin#) · B3 (Niacin, Nicotinamide#) · B5 (Pantothenic acid, Dexpanthenol, Pantethine) · B6 (Pyridoxine#, Pyridoxal phosphate, Pyridoxamine) · B7 (Biotin) · B9 (Folic acid, Dihydrofolic acid, Folinic acid) · B12 (Cyanocobalamin, Hydroxocobalamin, Methylcobalamin, Cobamamide) · CholineAscorbic acid# · Dehydroascorbic acidCombinations M: NUT
cof, enz, met
noco, nuvi, sysi/epon, met
drug(A8/11/12)
Categories:- Organic acids
- Neurology
Wikimedia Foundation. 2010.