- Methylcyclopentadienyl manganese tricarbonyl
-
Methylcyclopentadienyl manganese tricarbonyl 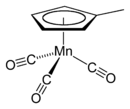
 tricarbonyl(methyl-η5-
tricarbonyl(methyl-η5-
cyclopentadienyl)manganeseOther namesMMTIdentifiers CAS number 12108-13-3 RTECS number OP1450000 Properties Molecular formula C9H7MnO3 Molar mass 218.09 g/mol Appearance pale yellow liquid
herbaceous odorDensity 1.38 g/cm³ Melting point -1 °C (272 K)
Boiling point 232-233 °C
Solubility in water low Solubility in other solvents alkane (petrol) Structure Coordination
geometryTetrahedral at Mn Hazards R-phrases 23/24/25-40 S-phrases 23-26-36/37/39-45 Main hazards flammable Flash point 205 °F Related compounds Related compounds ferrocene
Mn2(CO)10
dicyclopentadiene manganese tricarbonyl (verify) (what is:
manganese tricarbonyl (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methylcyclopentadienyl manganese tricarbonyl (MMT or MCMT) is an organomanganese compound with the formula (CH3C5H4)Mn(CO)3. Marketed initially in 1958 as a supplement to the gasoline additive tetraethyl lead to increase the fuel's octane rating, MMT was later used in unleaded gasoline.[1] Although banned as a gasoline additive in the United States from 1977 to 1995, MMT has been used in Canadian gasoline since 1976 (though was banned from 1997-1998 due to safety concerns)[2] and was recently introduced in Australia. It is sold under the tradenames HiTEC® 3000 and AK-33X.[3] It is also marketed as Ecotane by T2 Labs.[4]
Contents
History
Tetraethyl lead (TEL) has been, and still is, used as an additive to increase the octane rating of gasoline. TEL was manufactured and marketed by the Ethyl Corporation, a joint venture between General Motors and DuPont. When the US Environmental Protection Agency (EPA) ordered the phasing out of leaded gasoline in 1972, new fuel additives were sought. Initially marketed in 1958 as a smoke suppressant for gas turbines, MMT was used in 1974 as an additive in unleaded gasoline.
In 1977, use of MMT was banned in the US by the Clean Air Act until the Ethyl Corporation could prove that the additive would not lead to failure of new car emissions-control systems. As a result of this ruling, the Ethyl Corporation began a legal battle with the EPA, presenting evidence that MMT was harmless to automobile emissions-control systems. In 1995, the U.S. Court of Appeals ruled that the EPA had exceeded its authority and, as a result, MMT became a legal fuel additive in the US.[5] MMT is currently manufactured by the Afton Chemical Corporation, a subsidiary of Newmarket Corporation.[1] It is also produced and marketed as "Cestoburn" by Cestoil Chemical Inc. in Canada.
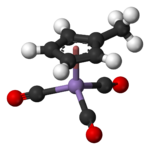
Structure and bonding
The compound is called a piano-stool or half-sandwich compound. The manganese atom in MMT is bonded to three carbonyl groups as well as to the methylcyclopentadienyl ring. These hydrophobic organic ligands make MMT highly lipophilic, which may increase bioaccumulation. A variety of related complexes are known, including ferrocene, which is also under consideration as an additive to gasoline.[citation needed]
Preparation
Related compounds
The related compound CpMn(CO)3 is also well studied as well as the corresponding thiocarbonyl derivatives CpMn(CS)2CO and CpMn(CS)(CO)2.[7]
Safety
The health considerations associated with MMT are divided into two areas; (1) MMT in its original chemical form and (2) the manganese combustion products emitted from vehicles operating on gasoline containing MMT as an octane improver.
1.MMT in its original chemical form
The chemical MMT is not accessible by the general public. Activities involving the neat chemical only occur at refineries or other industrial plants. In these cases, occupational regulations and the use of Personal Protective Equipment (PPE) limit exposure and risk to workers. A 2003 study by the NICNAS in Australia suggested that MMT in its raw concentrated form prior to its addition into gasoline was highly toxic.[8] Other studies have found the neat chemical to be cytotoxic and especially damaging to dopaminergic PC-12 cells[5] and Toxicokinetic studies have shown that manganese in the form of neat MMT absorbs into rat blood plasma at a rate 37 times that of inorganic manganese.[9]
Immediate symptoms of MMT exposure are mild skin and eye irritation. Short-term symptoms resulting from MMT poisoning by high exposures include giddiness, headache, nausea, and difficulties in breathing. Animal studies have shown that chronic exposure to MMT can result in damage to the liver and kidneys.[10] Chronic exposure to high levels of manganese, typically in certain occupational activities, has also been known to cause manganism, a disease with symptoms similar to those of Parkinson's disease. The chemical MMT is not accessible by the general public. Activities involving the neat chemical only occur at refineries or other industrial plants. In these cases, occupational regulations and the use of Personal Protective Equipment (PPE) limit exposure and risk to workers. Indeed, a 2002 study by Masashi Kitazawa argued that dermal absorption from accidental gasoline spills, misuse of gasoline as a solvent cleaner, and deliberate gasoline fume inhalation were the main sources of potential MMT exposure.
2.The manganese combustion products emitted from vehicles operating on gasoline containing MMT as an octane improver
The health hazards associated with manganese compounds emitted from vehicles operating on gasoline containing MMT as an octane improver have been debated for decades. In 1994 (reaffirmed in 1998, 2001 and 2010), Health Canada concluded that “airborne manganese resulting from the combustion of MMT in gasoline powered vehicles is not entering the Canadian environment in quantities or under conditions that may constitute a health risk”[11] and confirmed they were taking no action with respect to MMT. The 2003 NICNAS study ruled that the airborne concentrations of manganese as a result of car emissions from vehicles using fuel containing MMT posed no health hazard. Additional health studies, overseen by the US Environmental Protection Agency (EPA), have been conducted in order to explain the transport of manganese in the body. These studies, the most recent available, published by the Hamner Institutes for Health Sciences from 2007 through 2011 and submitted to the US Environmental Protection Agency (EPA) under the framework of the Clean Air Act, show that the body’s natural mechanisms can handle a wide range of manganese intake, whether from inhalation or ingestion.[12] While these mechanisms can be overwhelmed if exposures to manganese are very high (as in the case of some occupational exposures), the testing confirms that the body can safely handle inhaled manganese at, and well above, levels observed when MMT is used in gasoline. The studies also indicate that MMT use is safe for the entire population including vulnerable groups such as infants and the elderly. Although the EPA will now formally review the research findings, the US Agency recommended this data as meeting the Clean Air Act’s health study’s requirements and objectives.
Vehicle Manufacturer Recommendations
Before using MMT, you should first determine whether or not the manufacturer recommends use in the vehicle. Some manufacturers recommend against use of MMT in their vehicles.[13]
References
- ^ a b http://www.aftonchemical.com/Products/MMT/History+of+MMT.htm
- ^ The Globe and Mail "Threat of NAFTA Case Kills Canada's MMT Ban: Challenge over gasoline additive could have cost Ottawa millions". July 20, 1998
- ^ Frumkin, Howard and Solomon, Gena; "Manganese in the US Gas Supply" American Journal of Industrial Medicine, 31: 107-115 (1997)
- ^ "T2 Labs is the manufacturer of Ecotane". http://www.t2labs.com/ecotane/ecotane.htm. Retrieved December 20, 2007.
- ^ a b Masashi Kitazawa, Jarrad R. Wagner, Michael L. Kirby, Vellareddy Anantharam, and Anumantha G. Kanthasamy; Oxidative Stress and Mitochondrial-Mediated Apoptosis in Dopaminergic Cells Exposed to Methylcyclopentadienyl Manganese Tricarbonyl J. Pharmacol. Exp. Ther., Jul 2002; 302: 26 - 35.
- ^ U.S. Patent 4,946,975
- ^ Petz, W., "40 years of transition-metal thiocarbonyl chemistry and the related CSe and CTe compounds", Coordination Chemistry Reviews, 2008, 252, 1689-1733.doi:10.1016/j.ccr.2007.12.011.
- ^ National Industrial Chemicals Notification and Assessment Scheme; Methylcyclopentadienyl Manganese Tricarbonyl June 2003. [1]
- ^ Zheng W, Kim H, Zhao Q (April 2000). "Comparative toxicokinetics of manganese chloride and methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats". Toxicol. Sci. 54 (2): 295–301. doi:10.1093/toxsci/54.2.295. PMID 10774811. http://toxsci.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=10774811.
- ^ United Nations Environment Programme with the World Health Organization; Manganese; Geneva, 1981.
- ^ Petition to the Commissioner of the Environment and Sustainable Development, under section 22 of the Auditor General Act. July 2001.[2]
- ^ Methylcyclopentadienyl Manganese Tricarbonyl (MMT) Alternative Tier 2 Health Effects Test Program, Docket EPA-HQ-OAR-2004-0074.[3]
- ^ General Motors, "2011 Chevrolet Malibu Owners Manual", 2010. [4]
Categories:- Organomanganese compounds
- Carbonyl complexes
- Antiknock agents
- Cyclopentadienyl complexes
Wikimedia Foundation. 2010.

