- Methyl isocyanide
-
Methyl isocyanide 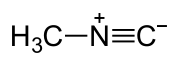
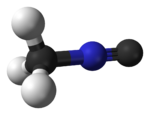 IsocyanomethaneOther namesmethyl isocyanide; isoacetonitrile; methylisonitrile
IsocyanomethaneOther namesmethyl isocyanide; isoacetonitrile; methylisonitrileIdentifiers CAS number 593-75-9 
PubChem 11646 ChemSpider 11156 
DrugBank DB04337 ChEBI CHEBI:44177 
Jmol-3D images Image 1 - [C-]#[N+]C
Properties Molecular formula C2H3N Molar mass 41.05 g mol−1 Appearance colorless liquid Density 0.786 g/mL liquid Melting point −45 °C
Boiling point 59-60 °C
Solubility in water miscible Solubility organic solvents Hazards EU classification Flammable, harmful R-phrases R11, R20/21/22, R36 S-phrases (S1/2), S16, S36/37 NFPA 704 Related compounds Related compounds acetic acid, acetamide, ethylamine, Acetonitrile  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl isocyanide or Isocyanomethane is an organic compound and a member of the isocyanide family. This colorless liquid is structurally similar to the isomeric methyl cyanide (acetonitrile), but its reactivity is very different. Methyl isocyanide is mainly used for making 5-membered heterocyclic rings or circlets.
Contents
Preparation
Methyl isocyanide was first prepared Gautier by reaction of silver cyanide with methyl iodide.[1][2] The common method for preparing methyl isocyanides is the dehydration of N-methylformamide.[3]
Uses
Methyl isocyanide is useful for the preparation of various heterocycles. It is also a ligand in organometallic chemistry.[4]
References
- ^ A. Gautier (1868). "Ueber eine neue Reihe von Verbindungen, welche mit den Cyanwasserstoffsäure-Aethern isomer sind". Ann. 146: 119–124. doi:10.1002/jlac.18681460107.
- ^ A. Gautier (1869). Ann. Chim. et Phys. 17 (103): 203.
- ^ Schuster, R. E.; Scott, J. E. (1973), "Methyl isocyanide", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0772
- ^ Heiner Eckert, Alfons Nestl, Ivar Ugi, "Methyl isocyanide" in Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons. doi:10.1002/047084289X.rm198
External links
Categories:- Hazardous air pollutants
- Isocyanides
- Solvents
Wikimedia Foundation. 2010.

