- Methyl hypochlorite
-
Methyl hypochlorite 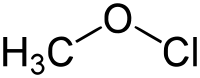 (Chlorooxy)methaneSystematic nameMethyl hypochloriteOther namesHypochlorous acid methyl ester; Methoxy chloride
(Chlorooxy)methaneSystematic nameMethyl hypochloriteOther namesHypochlorous acid methyl ester; Methoxy chlorideIdentifiers CAS number 593-78-2 
PubChem 79056 
ChemSpider 71388 
Jmol-3D images Image 1 - COCl
Properties Molecular formula CH3ClO Molar mass 66.49 g mol−1 Appearance Gas Odor Pungent Density 1.058 g/cm3 Melting point -120.4 °C, 153 K, -185 °F
Boiling point 9.18 °C, 282 K, 49 °F
Solubility in water Decomposes Refractive index (nD) 1.343 Hazards EU Index Not listed R-phrases R3 R8 R23/24/25 R35 NFPA 704  hypochlorite (verify) (what is:
hypochlorite (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl hypochlorite is an unstable and highly toxic compound that can be produced from reacting methanol with hypochlorous acid. It is extremely unstable and can decompose explosively giving off toxic fumes. Its instability is caused by the combination of the reducing methyl group and the oxidizing hypochlorite group.

This article about an organic compound is a stub. You can help Wikipedia by expanding it.

