- 2-Undecanone
-
2-Undecanone 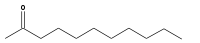 Undecan-2-oneOther namesMethyl nonyl ketone (MNK)
Undecan-2-oneOther namesMethyl nonyl ketone (MNK)
Nonyl methyl ketone
Methyldecananone
2-Hendecanone
Undecanone
IBI-246Identifiers CAS number 112-12-9 
ChemSpider 7871 
UNII YV5DSO8CY9 
DrugBank DB08688 KEGG C01875 
ChEBI CHEBI:17700 
RTECS number YQ2820000 Jmol-3D images Image 1 - O=C(CCCCCCCCC)C
Properties Molecular formula C11H22O Molar mass 170.29 g mol−1 Appearance Colorless or pale yellow liquid Density 0.829 g/cm³, liquid Melting point 15 °C, 288 K, 59 °F
Boiling point 231 °C, 504 K, 448 °F
Solubility in water 0.00179 g/100 mL (25 °C) Hazards MSDS External MSDS EU classification Flammable (F)
Irritant (Xi)R-phrases R50 R51 S-phrases S23 S24 S25 NFPA 704 Flash point 88 °C Related compounds Related Ketones Acetone
Butan-2-one
3-pentanone (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references 2-Undecanone, also known as methyl nonyl ketone and IBI-246, is an oily organic liquid manufactured synthetically, but which can also be extracted from oil of rue. It is found naturally in bananas, cloves, ginger, guava, strawberries, wild-grown tomatoes, and the perennial Houttuynia cordata.[1]
Contents
Uses
2-Undecanone is used in the perfumery and flavoring industries, but because of its strong odor it is primarily used as an insect repellent or animal repellent. Typically, 1–2% concentrations of 2-undecanone are found in dog and cat repellents in the form of a liquid, aerosol spray, or gel.
Research from North Carolina State University has shown that it may be useful as a mosquito repellent, as effective as, or even more effective than, DEET.[2][3]
Chemical properties
2-Undecanone is a ketone that is soluble in ethanol, benzene, chloroform, and acetone, but its large carbon chain renders it insoluble in water. Like most methyl ketones, 2-undecanone undergoes a haloform reaction when in the presence of a base and hypochlorite (a basic solution of hypochlorite). For example, the reaction between 2-undecanone and sodium hypochlorite yields sodium decanoate, chloroform, and sodium hydroxide.
- CH3CO(CH2)8CH3 + 3 NaOCl → CH3(CH2)8COONa + CHCl3 + 2 NaOH
See also
- Perfume allergy
Notes
- ^ Liang, Minmin et al.; Qi, M; Zhang, C; Zhou, S; Fu, R; Huang, J (2005). "Gas chromatography–mass spectrometry analysis of volatile compounds from Houttuynia cordata Thunb after extraction by solid-phase microextraction, flash evaporation and steam distillation". Analytica Chimica Acta 531 (1): 97–104. doi:10.1016/j.aca.2004.09.082.
- ^ Mosquitoes Repelled By Tomato-Based Substance; Safer, More Effective Than DEET, Science Daily, June 2002
- ^ Stephen J. Toth, Jr. and Wayne G. Buhler (2002). North Carolina State University Scientist Discovers Mosquito Repellent in Tomatoes. 12. http://ipm.ncsu.edu/current_ipm/Broadcasts/broadcast06-02b.html.
References
- Lange's Handbook of Chemistry (14th Edition), McGraw-Hill, 1992; Section 1; Table 1.15
- The Condensed Chemical Dictionary (10th Edition), Gesner G. Hawley
- 2-Undecanone from The Good Scents Company
- MSDS for 2-Undecanone
Categories:- Ketones
- Insect repellents
Wikimedia Foundation. 2010.

