- Methyl azide
-
Methyl azide 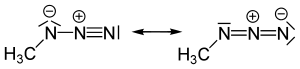 Other namesazidomethane
Other namesazidomethaneIdentifiers PubChem 79079 ChemSpider 71411 
Jmol-3D images Image 1 - [N-]=[N+]=N\C
Properties Molecular formula CH3N3 Molar mass 57.05 Explosive data Shock sensitivity High Friction sensitivity High Hazards Main hazards Harmful, Explosive Related compounds Related compounds Hydrazoic acid, Chlorine azide, Ethyl azide  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl azide CH3N3, is a covalent molecule related to hydrazoic acid and other alkyl azides. Methyl azide is a linear molecule. In resonance the central nitrogen is clearly linear (two electron groups), but the nitrogen bonded to H3C, has three electron groups in H3C-N=N+=N- ⇔ H3C-N--N+≡N . [1]
It can be prepared by a methylation of sodium azide. The first synthesis was reported in 1905. [2] It decomposes in a first-order reaction CH3N3 → CH3N + N2 [3]
Methyl azide might be a potential precursor in the synthesis of prebiotic molecules via nonequilibrium reactions on interstellar ices initiated by energetic galactic cosmic rays (GCR) and photons. [4]
Safety Precautions
Methyl azide is stable at ambient temperature but may explode when heated. [5] Presence of mercury increases the sensitivity to shock and spark. Incompatible with (dimethyl malonate + sodium methylate); mercury; methanol; sodium azide; dimethyl sulfate; sodium hydroxide; hydrogen azide. When heated to decomposition it emits toxic fumes of NOx.
References
- ^ Pauling, Linus, Brockway, O.; The Adjacent Charge Rule and the Structure of Methyl Azide, Methyl nitrate, and Fluorine nitrate; JACS, 1937, 59 (1), pp 13-20 DOI:10.1021/ja01280a005
- ^ Dimroth, O. and Wislicenus, W.; Chemische Berichte ; 38, 1573 (1905)
- ^ O'Dell, M.S. and Darwent B. Thermal decomposition of methyl azideCanadian Journal of Chemistry, 48, 1140 (1970)
- ^ Alfredo Quinto-Hernandez and Alec M. Wodtke, Department of Chemistry and Biochemistry, University of California, Santa Barbara; Chris J. Bennett, Y. Seol Kim, and Ralf I. Kaiser; Department of Chemistry, University of Hawaii at Manoa; On the Interaction of Methyl Azide (CH3N3) Ices with Ionizing Radiation: Formation of Methanimine (CH2NH), Hydrogen Cyanide (HCN), and Hydrogen Isocyanide (HNC) ; Journal of Physical Chemistry DOI: 10.1021/jp103028v Publication Date (Web): December 17, 2010
- ^ Bretherick's handbook of reactive chemical hazards; edited by P. G. Urben
- Graner, G., Hirota, E., Iijima, T., Kuchitsu, K., Ramsay,D. A., Vogt, J., Vogt,N.: CH3N3 Methyl azide. Kuchitsu, K. (ed.). SpringerMaterials - The Landolt-Börnstein Database [1] DOI: 10.1007/10653318_320
- NIST Webbook
Categories:- Azides
- Explosive chemicals
Wikimedia Foundation. 2010.
