- n-Propyl azide
-
n-Propyl azide 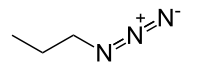 1-Azidopropane
1-AzidopropaneIdentifiers CAS number 22293-25-0 
Jmol-3D images Image 1 - [N-]=[N+]=NCCC
Properties Molecular formula C3H7N3 Molar mass 85.11 g mol−1 Hazards Main hazards Harmful, Explosive Related compounds Related compounds Hydrazoic acid, Chlorine azide, Ethyl azide  azide (verify) (what is:
azide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references n-Propyl azide is a covalent molecule related to hydrazoic acid and other alkyl azides.[1]
n-Propyl azide has been used in the laboratory synthesis of pharmaceutical drug candidates.[2][3]
References
- ^ Stefan Bräse (Editor), Klaus Banert (Co-Editor); Organic Azides: Syntheses and Applications; 2010 John Wiley and Sons; ISBN 978-0-470-51998-1
- ^ Helmut Haning et al (2005). "Comparison of different heterocyclic scaffolds as substrate analog PDE5 inhibitors". Bioorganic & Medicinal Chemistry Letters 15 (17): 3900–3907.
- ^ Michael H. Parker et al (2002). "Synthesis of (-)-5,8-Dihydroxy-3R-methyl-2R-(dipropylamino)-1,2,3,4-tetrahydronaphthalene: An Inhibitor of β-Amyloid1-42 Aggregation". Bioorganic & Medicinal Chemistry 10 (11): 3565–3569.
Further reading
- Edward J. Kaufmann, Richard C. Thompson (1977). "Reduction of organic azides by chromium(II) in aqueous solution". JACS 99 (6): 1824–1830. doi:10.1021/ja00448a025.

This article about an organic compound is a stub. You can help Wikipedia by expanding it.
