- Chlorine azide
-
Chlorine azide 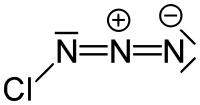 Other namesChlorine nitride; Nitrogen chloride
Other namesChlorine nitride; Nitrogen chlorideIdentifiers PubChem 61708 Jmol-3D images Image 1 - [N-]=[N+]=NCl
Properties Molecular formula ClN3 Molar mass 77.4731 Appearance yellow orange liquid Melting point freezes at -100C
Boiling point approx -15C
Solubility soluble in butane, pentane, benzene, methanol, ethanol, diethyl ether, acetone, chloroform, carbon tetrachloride, and carbon disulfide; slightly soluble in water Explosive data Shock sensitivity High Friction sensitivity High Hazards Main hazards Harmful, Explosive Related compounds Related compounds Hydrazoic acid  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Chlorine azide (ClN3) is an inorganic compound. It was discovered by Friedrich Raschig in 1908.[1] More so than other azides, ClN3 is highly explosive. In undiluted condition, it usually detonates violently whatever the temperature, without apparent provocation.[2]
Contents
Preparation and handling
Chlorine azide is prepared by passing chlorine gas over silver azide or by an addition of acetic acid to a solution of sodium hypochlorite and sodium azide.[3]
When treated with ammonia it is conceivable that one or more of the three possible azinamines, NH2N3, NH(N3)2, and N(N3)3 may be formed.
Explosive characteristics
Chlorine azide is highly sensitive. It will explode without provocation and is too sensitive to be used commercially. Explosive reaction with 1,3-butadiene, ethane, ethylene, methane, propane, phosphorus, silver azide, sodium. Reacts with water or steam to produce toxic and corrosive fumes of hydrochloric acid.[4]
Regulatory information
US Department of Transportation forbids shipment.
References
- ^ Frierson, Joe; Browne, A. W.; Chlorine Azide. II. Interaction of Chlorine Azide and Silver Azide. Azino Silver Chloride, N3AgCl JACS, 1943, 65 (9), pp1698–1700 DOI: 10.1021/ja01249a013 Publication Date: September 1943
- ^ Frierson, W. Joe; Kronrad, J.; Browne, A. W.; Chlorine Azide, ClN3, JACS., 1943, 65 (9), pp 1696–1698 DOI: 10.1021/ja01249a012 Publication Date: September 1943
- ^ Raschig, F. (1908). "Über Chlorazid N3 Cl". Berichte der deutschen chemischen Gesellschaft 41 (3): 4194–4195. doi:10.1002/cber.190804103130.
- ^ Pubchem
Categories:- Azides
- Explosive chemicals
- Inorganic compounds
Wikimedia Foundation. 2010.
