- Ethane
-
Ethane 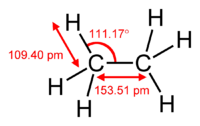
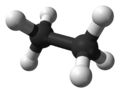
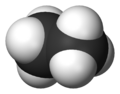 Ethane
EthaneIdentifiers CAS number 74-84-0 
PubChem 6324 ChemSpider 6084 
UNII L99N5N533T 
EC number 200-814-8 UN number 1035 ChEBI CHEBI:42266 
ChEMBL CHEMBL135626 
RTECS number KH3800000 Jmol-3D images Image 1 - CC
Properties Molecular formula C2H6 Molar mass 30.07 g/mol Appearance colourless gas Density 1.282 kg/m3(15 C gas, 1 atm)
546.49 kg/m3(liquid, at b.p.,1 atm)Melting point Boiling point -89 °C (184 K)
Solubility in water very low Acidity (pKa) 50 Hazards MSDS External MSDS EU Index 601-002-00-X EU classification Highly flammable (F+) R-phrases R12 S-phrases (S2), S9, S16, S33 NFPA 704 Flash point -135 °C Autoignition
temperature472 °C Explosive limits 3.0–12.5% Related compounds Related alkanes Methane
PropaneSupplementary data page Structure and
propertiesn, εr, etc. Thermodynamic
dataPhase behaviour
Solid, liquid, gasSpectral data UV, IR, NMR, MS  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas.
Ethane is isolated on an industrial scale from natural gas, and as a byproduct of petroleum refining. Its chief use is as petrochemical feedstock for ethylene production.
Contents
History
Ethane was first synthetically created in 1834 by Michael Faraday, applying electrolysis of a potassium acetate solution. He mistook the hydrocarbon product of this reaction for methane, and did not investigate it further.[1] During the period 1847–1849, in an effort to vindicate the radical theory of organic chemistry, Hermann Kolbe and Edward Frankland produced ethane by the reductions of propionitrile (ethyl cyanide)[2] and ethyl iodide[3] with potassium metal, and, as did Faraday, by the electrolysis of aqueous acetates. They, however, mistook the product of these reactions for methyl radical, rather than the dimer of methyl, ethane. This error was corrected in 1864 by Carl Schorlemmer, who showed that the product of all these reactions was in fact ethane.
The name ethane is derived from the IUPAC nomenclature of organic chemistry. "Eth-" refers to the presence of 2 carbon atoms, and "-ane" refers to the presence of a single bond between them.
Chemistry
In the laboratory, ethane may be conveniently prepared by Kolbe electrolysis. In this technique, an aqueous solution of an acetate salt is electrolysed. At the anode, acetate is oxidized to produce carbon dioxide and methyl radicals, and the highly reactive methyl radicals combine to produce ethane:
Another method, the oxidation of acetic anhydride by peroxides, is conceptually similar.
The chemistry of ethane also involves chiefly free radical reactions. Ethane can react with the halogens, especially chlorine and bromine, by free radical halogenation. This reaction proceeds through the propagation of the ethyl radical:
Because halogenated ethanes can undergo further free radical halogenation, this process results in a mixture of several halogenated products. In the chemical industry, more selective chemical reactions are used for the production of any particular two-carbon halocarbon.
Combustion
The complete combustion of ethane releases 1559.7 kJ/mol, or 51.9 kJ/g, of heat, and produces carbon dioxide and water according to the chemical equation
Combustion occurs by a complex series of free-radical reactions. Computer simulations of the chemical kinetics of ethane combustion have included hundreds of reactions. An important series of reaction in ethane combustion is the combination of an ethyl radical with oxygen, and the subsequent breakup of the resulting peroxide into ethoxy and hydroxyl radicals.
- C2H5• + O2 → C2H5OO•
- C2H5OO• + HR → C2H5OOH + •R
- C2H5OOH → C2H5O• + •OH
The principal carbon-containing products of incomplete ethane combustion are single-carbon compounds such as carbon monoxide and formaldehyde. One important route by which the carbon-carbon bond in ethane is broken to yield these single-carbon products is the decomposition of the ethoxy radical into a methyl radical and formaldehyde, which can in turn undergo further oxidation.
- C2H5O• → CH3• + CH2O
Some minor products in the incomplete combustion of ethane include acetaldehyde, methane, methanol, and ethanol. At higher temperatures, especially in the range 600–900 °C, ethylene is a significant product. It arises via reactions like
Similar reactions (although with species other than oxygen as the hydrogen abstractor) are involved in the production of ethylene from ethane in steam cracking.
Ethane Barrier
Rotating a molecular substructure about a twistable bond usually requires energy. The minimum energy to produce a 360-degree bond rotation is called the rotational barrier.
Ethane gives a classic, simple example of such a rotational barrier, sometimes called the "ethane barrier." The three hydrogens at each end are free to pinwheel about the central carbon-carbon bond, provided that there is sufficient energy to overcome the barrier of the carbon-hydrogen bonds at each end of the molecule bumping into each other by way of overlap (exchange) repulsion [4] (see diagram at right).
As far back as 1890-1891 ethane was suggested to prefer a staggered conformation with the two ends of the molecule askew from each other. [5] [6] [7]
Production
After methane, ethane is the second-largest component of natural gas. Natural gas from different gas fields varies in ethane content from less than 1% to over 6% by volume. Prior to the 1960s, ethane and larger molecules were typically not separated from the methane component of natural gas, but simply burnt along with the methane as a fuel. Today, however, ethane is an important petrochemical feedstock, and it is separated from the other components of natural gas in most well-developed gas fields. Ethane can also be separated from petroleum gas, a mixture of gaseous hydrocarbons that arises as a byproduct of petroleum refining. Economics of building and running processing plants can change, however. If the relative value of sending the unprocessed natural gas to a consumer exceeds the value of extracting ethane, then the plant may not be run. This can cause operational issues managing the changing quality of the gas in downstream systems.[1]
Ethane is most efficiently separated from methane by liquefying it at cryogenic temperatures. Various refrigeration strategies exist: the most economical process presently in wide use employs turboexpansion, and can recover over 90% of the ethane in natural gas. In this process, chilled gas expands through a turbine; as it expands, its temperature drops to about -100 °C. At this low temperature, gaseous methane can be separated from the liquefied ethane and heavier hydrocarbons by distillation. Further distillation then separates ethane from the propane and heavier hydrocarbons
Uses
The chief use of ethane is in the chemical industry in the production of ethene (ethylene) by steam cracking. When diluted with steam and briefly heated to very high temperatures (900 °C or more), heavy hydrocarbons break down into lighter hydrocarbons, and saturated hydrocarbons become unsaturated. Ethane is favored for ethene production because the steam cracking of ethane is fairly selective for ethene, while the steam cracking of heavier hydrocarbons yields a product mixture poorer in ethene, and richer in heavier alkenes (olefins) such as propene (propylene) and butadiene, and in aromatic hydrocarbons.
Experimentally, ethane is under investigation as a feedstock for other commodity chemicals. Oxidative chlorination of ethane has long appeared to be a potentially more economical route to vinyl chloride than ethene chlorination. Many processes for carrying out this reaction have been patented, but poor selectivity for vinyl chloride and corrosive reaction conditions (specifically, a hydrochloric acid-containing reaction mixture at temperatures greater than 500 °C) have discouraged the commercialization of most of them. Presently, INEOS operates a 1000 t/a ethane-to-vinyl chloride pilot plant at Wilhelmshaven in Germany.
Similarly, the Saudi Arabian firm SABIC has announced construction of a 30,000 t/a plant to produce acetic acid by ethane oxidation at Yanbu. This economic viability of this process may rely on the low cost of ethane near Saudi oil fields, and it may not be competitive with methanol carbonylation elsewhere in the world.
Ethane can be used as a refrigerant in cryogenic refrigeration systems. On a much smaller scale, in scientific research, liquid ethane is used to vitrify water-rich samples for electron microscopy. A thin film of water, quickly immersed in liquid ethane at -150 °C or colder, freezes too quickly for water to crystallize. This rapid freezing does not disrupt the structure of soft objects present in the liquid state, as the formation of ice crystals can do.
Health and safety
At room temperature, ethane is a flammable gas. When mixed with air at 3.0% – 12.5% by volume, it forms an explosive mixture.
Some additional precautions are necessary where ethane is stored as a cryogenic liquid. Direct contact with liquid ethane can result in severe frostbite. In addition, the vapors evaporating from liquid ethane are, until they warm to room temperature, heavier than air and can creep along the ground or gather in low places, and if they encounter an ignition source, can flash back to the body of ethane from which they evaporated.
Containers recently emptied of ethane may contain insufficient oxygen to support life. Beyond this asphyxiation hazard, ethane poses no known acute or chronic toxicological risk. It is not known or suspected to be a carcinogen.
Atmospheric and extraterrestrial ethane
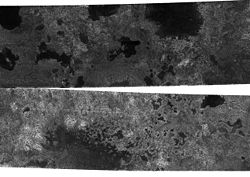 A photograph of Titan's northern latitudes. The dark features appear to be hydrocarbon lakes, but further images will be needed to see if the dark spots remain the same (as they would if they were lakes)
A photograph of Titan's northern latitudes. The dark features appear to be hydrocarbon lakes, but further images will be needed to see if the dark spots remain the same (as they would if they were lakes)
Ethane occurs as a trace gas in the Earth's atmosphere, currently having a concentration at sea level of 0.5 ppbv,[8] though its pre-Industrial concentration is likely to have been lower since a significant proportion of the ethane in today's atmosphere may have originated as fossil fuels. Although ethane is a greenhouse gas, it is much less abundant than methane and also less efficient relative to mass. It has also been detected as a trace component in the atmospheres of all four giant planets, and in the atmosphere of Saturn's moon Titan.
Atmospheric ethane results from the Sun's photochemical action on methane gas, also present in these atmospheres: ultraviolet photons of shorter wavelengths than 160 nm can photo-dissociate the methane molecule into a methyl radical and a hydrogen atom. When two methyl radicals recombine, the result is ethane:
- CH4 → CH3• + •H
- CH3• + •CH3 → C2H6
In the case of Titan, it was once widely hypothesized that ethane produced in this fashion rained back onto the moon's surface, and over time had accumulated into hydrocarbon seas or oceans covering much of the moon's surface. Infrared telescopic observations cast significant doubt on this hypothesis, and the Huygens probe, which landed on Titan in 2005, failed to observe any surface liquids, although it did photograph features that could be presently dry drainage channels. In December 2007 the Cassini probe found at least one lake at Titan's south pole, now called Ontario Lacus because of the lake's similar area to Lake Ontario on Earth (approximately 20,000 km²). Further analysis of infrared spectroscopic data presented in July 2008[9] provided stronger evidence for the presence of liquid ethane in Ontario Lacus.
In 1996, ethane was detected in Comet Hyakutake,[10] and it has since been detected in some other comets. The existence of ethane in these distant solar system bodies may implicate ethane as a primordial component of the solar nebula from which the sun and planets are believed to have formed.
In 2006, Dale Cruikshank of NASA/Ames Research Center (a New Horizons co-investigator) and his colleagues announced the spectroscopic discovery of ethane on Pluto's surface.[11]
References
- ^ (Faraday 1834)
- ^ (Kolbe & Frankland 1849)
- ^ (Frankland 1850)
- ^ Pitzer RM, "The barrier to internal rotation in ethane". Accts. chem. Res. 16, 201-210 (1983)
- ^ Bischoff, CA, (1890), Chem. Ber. 23, 623
- ^ Bischoff, CA, (1891a), Chem. Ber. 24, 1074, 1086
- ^ Bischoff, CA, (1891b), Chem. Ber. 26, 1452
- ^ Trace gases
- ^ The identification of liquid ethane in Titan/'s Ontario Lacus : Abstract : Nature
- ^ (Mumma 1996)
- ^ A. Stern (November 1, 2006). "Making Old Horizons New". The PI's Perspective. Johns Hopkins University Applied Physics Laboratory. http://pluto.jhuapl.edu/overview/piPerspectives/piPerspective_11_1_2006.php. Retrieved 2007-02-12.
Sources
- Faraday, Michael (1834), "Experimental researches in electricity: Seventh series", Philosophical Transactions 124: 77–122, doi:10.1098/rstl.1834.0008
- Kolbe, Hermann; Frankland, Edward (1849), "On the products of the action of potassium on cyanide of ethyl", Journal of the Chemical Society 1: 60–74
- Frankland, Edward (1850), "On the isolation of the organic radicals", Journal of the Chemical Society 2: 263–296
- Kolbe, Hermann (1850), "Researches on the electrolysis of organic compounds", Journal of the Chemical Society 2: 157–184
- Schorlemmer, Carl (1864), Annalen der Chimie 132: 234
- Mumma, Michael J.; et al. (1996), "Detection of Abundant Ethane and Methane, Along with Carbon Monoxide and Water, in Comet C/1996 B2 Hyakutake: Evidence for Interstellar Origin", Science 272 (5266): 1310–1314, Bibcode 1996Sci...272.1310M, doi:10.1126/science.272.5266.1310, PMID 8650540
- Brown, Bob; et al. (2008), NASA Confirms Liquid Lake on Saturn Moon, http://www.jpl.nasa.gov/news/news.cfm?release=2008-152
External links
- International Chemical Safety Card 0266
- Market-Driven Evolution of Gas Processing Technologies for NGLs
- Staggered and eclipsed ethane
Alkanes Higher alkanes · List of alkanes Categories:- Alkanes
Wikimedia Foundation. 2010.


