- Methyl vinyl ether
-
Methyl vinyl ether 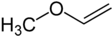 Other namesMethoxyethene, ethenyl methyl ether, vinyl methyl ether
Other namesMethoxyethene, ethenyl methyl ether, vinyl methyl etherIdentifiers CAS number 107-25-5 PubChem 7861 ChemSpider 7573 
Jmol-3D images Image 1
Image 2- O(C=C)C
COC=C
Properties Molecular formula C3H6O Molar mass 58.08 g mol−1 Density 0.77 g/cm–3[1] Melting point –122 °C[1]
Boiling point 6 °C[1]
Vapor pressure 157 kPa (20 °C)[1] Hazards EU classification  F+
F+R-phrases R12 S-phrases (S2), S9, S16, S33 Flash point –60 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Methyl vinyl ether is an organic compound with the chemical formula C3H6O. It is the simplest chemical that contains both an alkene group and an alkyl group with an ether linkage. The compound can be made by reaction of acetylene and methanol in presence of a base. [2]
The alkene portion of the molecule is reactive in many ways. It is prone to polymerization, leading to formation of poly(methyl vinyl ether) (PMVE, CAS: 9003-09-2). This mode of reactivity is analogous to the way vinyl acetate and vinyl chloride can be polymerized to form polyvinyl acetate and polyvinyl chloride, respectively. The alkene can also react in [4+2] cycloaddition reactions.[3] The reaction of it with acrolein is the first step in the commercial synthesis of glutaraldehyde.
The compound is useful as a synthon for nucleophilic acylation, via deprotonation of the alkene adjacent to the oxygen attachment.[4] In particular, this approach allows synthesis of a variety of acyl derivatives of silicon, germanium, and tin that cannot be made easily by other routes.[5][6][7]
References
- ^ a b c d Record of Methylvinylether in the GESTIS Substance Database from the IFA
- ^ Oxygenated fuel additives: The formation of methyl vinyl ether and 1,1-dimethoxyethane by the catalysed reaction of acetylene with methanol David Trimma, Corresponding Author Contact Information, E-mail The Corresponding Author, Noel Cantb and Yun Leib Catalysis Today Volume 145, Issues 1-2, 15 July 2009, Pages 163-168 doi:10.1016/j.cattod.2008.04.015
- ^ Longley, Jr., R. I.; Emerson, W. S. (1950). "The 1,4-Addition of Vinyl Ethers to α,β-Unsaturated Carbonyl Compounds". J. Am. Chem. Soc. 72 (7): 3079–3081. doi:10.1021/ja01163a076.
- ^ Lever, Jr., O. W. (1976). "New horizons in carbonyl chemistry: reagents for nucleophilic acylation". Tetrahedron 32: 1943–1971. doi:10.1016/0040-4020(76)80088-9.
- ^ Soderquist, J. A.; Hassner, A. (1980). "Synthetic methods. 15. Unsaturated acyl derivatives of silicon, germanium, and tin from metalated enol ethers". J. Am. Chem. Soc. 102: 1577–1583. doi:10.1021/ja00525a019.
- ^ Soderquist, J. A.; Hassner, A. (1980). "Vinylmetalloids. 3. Sila- and germacyclopentan-2-ones from metallated enol ethers". J. Org. Chem. 45: 541–543. doi:10.1021/jo01291a041.
- ^ Soderquist, J. A. (1990), "Acetyltrimethylsilane", Org. Synth. 68: 25, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv8p0019; Coll. Vol. 8: 19
Categories:- Ethers
- Alkenes
- O(C=C)C
Wikimedia Foundation. 2010.
