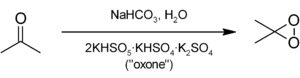- Dimethyldioxirane
-
Dimethyldioxirane 
 3,3-DimethyldioxiraneOther namesDMDO
3,3-DimethyldioxiraneOther namesDMDOIdentifiers CAS number 74087-85-7 
PubChem 115197 Jmol-3D images Image 1 - CC1(OO1)C
Properties Molecular formula C3H6O2 Molar mass 74.08 g/mol  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dimethyldioxirane (DMDO) is a dioxirane derived from acetone. It is the most commonly used dioxirane in organic synthesis.
Synthesis
DMDO is not commercially available because of its instability. DMDO can be prepared by the reaction of acetone with oxone, where the potassium peroxymonosulfate is the active ingredient:[1]
The preparation of DMDO is rather inefficient (typical yields < 3%) and typically only yields a relatively dilute solution in acetone (approximately 0.15 M). However, this is of no consequence, since DMDO is prepared from extremely cheap starting materials: acetone, sodium bicarbonate, and potassium peroxymonosulfate (commercially known as "oxone"). A freshly prepared solution of DMDO in acetone will last approximately one to two weeks in the freezer. Frequent titrations (typically by 1H NMR with thioanisole) are required.[citation needed]
Uses
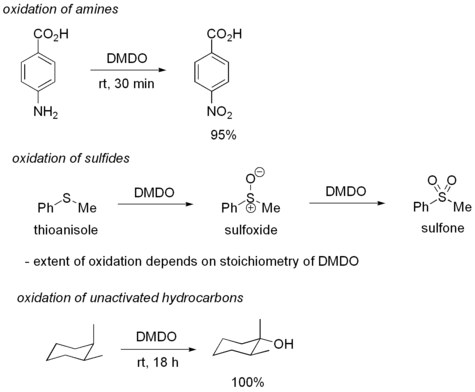
The most common use for DMDO is the oxidation of alkenes to epoxides. One particular advantage of using DMDO is that the only byproduct of oxidation is acetone, a fairly innocuous and volatile compound. DMDO oxidations are particularly mild, sometimes allowing oxidations which might not otherwise be possible. In fact, DMDO is considered the reagent of choice for epoxidation, and in nearly all circumstances is as good as or better than peroxyacids such as meta-Chloroperoxybenzoic acid (m-CPBA).
Despite its high reactivity, DMDO displays good selectivity for olefins. Typically, electron deficient olefins are oxidized more slowly than electron rich ones. DMDO will also oxidize several other functional groups. For example, DMDO will oxidize primary amines to nitro compounds and sulfides to sulfoxides. In some cases, DMDO will even oxidize unactivated C-H bonds:
DMDO can also be used to convert nitro compounds to carbonyl compounds (Nef reaction).
References
- ^ Robert W. Murray and Megh Singh (1988), "Synthesis of epoxides using dimethyldioxirane: trans-stilbene oxide"], Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv9p0288; Coll. Vol. 9: 288
- Dimethyldioxirane. Crandall, J.K.; Curci, R.; D'Accolti, L.; Fusco, C. Encyclopedia of Reagents for Organic Synthesis (2005). DOI: 10.1002/047084289X.rd329
Categories:- Reagents for organic chemistry
- Organic peroxides
- Dioxiranes
Wikimedia Foundation. 2010.