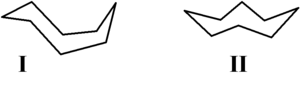- Cyclooctane
-
Cyclooctane 

Identifiers CAS number 292-64-8 
PubChem 9266 ChemSpider 8909 
ChEMBL CHEMBL452651 
Jmol-3D images Image 1 - C1CCCCCCC1
Properties Molecular formula C8H16 Molar mass 112.21 g/mol Density 0.834 g/cm3 Melting point 14.59 °C
Boiling point 149 °C
Solubility in water 7.90 mg/L Related compounds Related cycloalkanes Cycloheptane  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Cyclooctane is a cycloalkane with the molecular formula (CH2)8.[1] It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general.
Conformation
The conformation has been studied extensively using computational methods. Hendrickson noted that "cyclooctane is unquestionably the conformationally most complex cycloalkane owing to the existence of many conformers of comparable energy." The boat-chair conformation I is the most stable form.[2] This conformation was confirmed by Allinger and co-workers.[3] The crown conformation[4] II is slightly less stable. Among the many compounds exhibiting the crown conformation (structure II) is S8, elemental sulfur.
Synthesis and reactions
The main route to cyclooctane derivatives involves the dimerization of butadiene, catalysed by nickel(0) complexes such as nickel bis(cyclooctadiene).[5] This process affords, among other products, 1,5-cyclooctadiene (COD), which can be hydrogenated. COD is widely used for the preparation of precatalysts for homogeneous catalysis. The activation of these catalysts under H2, produces cyclooctane, which is usually discarded or burnt:
- C8H12 + 2 H2 → C8H16
Cyclooctane participates in no reactions except those typical of a other saturated hydrocarbons, combustion and free radical halogenation. Recent work on alkane functionalisation, using peroxides such as dicumyl peroxide, has opened up the chemistry to some extent, allowing for example the introduction of a phenylamino group.[6]
References
- ^ Mackay, Donald (2006). Handbook of Physical-chemical Properties and Environmental Fate for Organic Chemicals. CRC Press. p. 258. ISBN 1566706874. http://books.google.com/?id=wjd-nEugVskC.
- ^ Hendrickson, James B. (1967). "Molecular Geometry V. Evaluation of Functions and Conformations of Medium Rings". Journal of the American Chemical Society 89 (26): 7036–7043. doi:10.1021/ja01002a036
- ^ Dorofeeva, O.V.; Mastryukov, V.S.; Allinger, N.L.; Almenningen, A. (1985). "The molecular structure and conformation of cyclooctane as determined by electron diffraction and molecular mechanics calculations". The Journal of Physical Chemistry 89 (2): 252–257. doi:10.1021/j100248a015. http://pubs.acs.org/doi/pdf/10.1021/j100248a015. Retrieved 2008-02-05.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "crown conformation".
- ^ Thomas Schiffer, Georg Oenbrink “Cyclododecatriene, Cyclooctadiene, and 4-Vinylcyclohexene” in Ullmann’s Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim.doi:10.1002/14356007.a08_205
- ^ Deng, Guojun; Wenwen Chen, Chao-Jun Li (February 2009). "An Unusual Peroxide-Mediated Amination of Cycloalkanes with Nitroarenes". Advanced synthesis & catalysis 351 (3): 353–356. doi:10.1002/adsc.200800689.
Cyclopropane · Cyclobutane · Cyclopentane · Cyclohexane · Cycloheptane · Cyclooctane · Cyclononane · Cyclodecane · Cycloundecane · CyclododecaneCategories:- Cycloalkanes
- Hydrocarbon solvents
Wikimedia Foundation. 2010.